Abstract
Antibacterial activities of aqueous, acetone, ethanol and methanol extracts of fruits of Helicteres isora (Mororphali) were studied. The fruit aqueous extracts of H. isora showed prominent antibacterial activities against E.coli, Staphylococcus epidermidis, Salmonella typhimurium and Proteus vulgaris; moderate activity against Enterobacter aerogenes, Staphylococcus aureus, Salmonella typhi and least activity against Pseudomonas aeruginosa. The aqueous extract showed maximal, the ethanol and methanol extract moderate and acetone extracts least antibacterial activities. Phytochemical screening revealed the presence of carbohydrates, anthraquinon glycosides, proteins, tannin and phenolic compounds and steroids These antibacterial properties supports its traditional use of fruits of H. isora in the treatment of enteric or diarrhoeal infections.
Keywords: Antimicrobial activity, Helicteres isora, enteric pathogens, E.coli, Salmonella typhi, Pseudomonas aeruginosa
Introduction
In India, use of different parts of several medicinal plants to cure specific ailments has been in vogue from ancient times and inherited traditionally. The fruits of Helicteres isora Linn (Sterculiaceae) have been used in the indigenous system of medicine in India for the treatment of griping bowels and diarrheal diaseases (Krishnaraju et al, 2006). The roots and the bark are expectorant, demulcent, hypoglycemic and useful in colic, scabies, gastropathy, diabetes, diarrhoea and dysentery (Singh et al, 1985, Kirtikar and Basu, 1995, Prajapathi et al, 2003, Kumar et al, 2006). The fruits are astringents, refrigerant, stomatic, vulnerary and useful in griping of bowels, flatulence of children and antispasmodic (Chopra et al, 1956, Pohocha and Grampurohit, 2001). The barks of H.isora showed prominent antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli (Badgujar et al, 2006) and fruits against Candida albicans (Bonjar, 2004). The presence of flavones, triterpenoids, cucurbitacin, phytosterols, saponins, sugars and phlobatannins were demonstrated in roots and barks H.isora L. (Bean et al, 1985, Satake et al, 1999, Kumar et al. 2007).
Enteric or diarrheal infections are major public health problems in developing countries and contribute to the death of 3.3 to 6.0 million children annually. Enteric bacteria comprised of Salmonella spp., Shigella spp., Proteus spp., Klebsiella spp., E. coli, Pseudomonas spp., Vibrio cholerae and Staphylococcus aureus which are major etiologic agents of sporadic and epidemic diarrhea both in children and adults ((WHO, 1985, Ballal, 2005). W.H.O. (1993) reported that 80% populations rely mainly on traditional therapies, involving the use of plant extracts or their active constituents. The use of medicinal plants in India contributes significantly in primary health care and it is interesting to determine whether actual pharmacological effects support the traditional uses or merely based on folklore. The review revealed that the fruits of H.isora L. were used in diarrhoeal infection and it is anti-candidial but so far no information on antibacterial activities of fruits of H. isora is available hence, attempt was made to find out phytochemical contents and antibacterial potentials of fruits of H.isora against diarrhoeal/enteric bacterial pathogens.
Materials and methods
The fruits of Helicteres isora L. were collected in the forest of Melghat in Amravati district, India and authenticated by P.G. Department of Botany, S.G.B. Amravati University Amravati. The dried fruits of H. isora were washed with water, HgCl2 (0.5%), sterile distilled water and ground in to fine powder with auto-mix blender. The 20 g fine powder was suspended in 200ml of water or various organic solvents (methanol, ethanol, acetone) and extracted in soxhlet apparatus and vacuum dried. Amount of dry recovered powder extract was recorded (per 20 g of original fruit powder). This dry mass of various extracts H. isora L served for experimentations.
The presence of saponins, tannins, anthraquinones, alkaloids, triterpens, flvonoids, glycosides, reduced sugar and phlobatannins were detected by simple qualitative methods (Khandelwal, 2001).
Bacterial cultures
The standard pathogenic bacterial cultures were procured from IMTECH, Chandigarh, India and used in the present study (Table 1). The bacteria rejuvenated in Mueller-Hinton broth (Hi-media laboratories, Mumbai, India) at 37°C for 18 hrs and then stocked at 4°C in Mueller-Hinton Agar. Subcultures were prepared from the stock for bioassay. A loopful of culture was inoculated in 10 mL of sterile nutrient broth and incubated at 37° C for 3 hrs. Turbidity of the culture was standardized to 105 CFU with the help of SPC and turbidometer.
Table 1.
Bacterial cultures used in study (IMTECH, Chandigarh, India)
| Bacterial Pathogens | MTCC number |
| Escherichia coli (E.coli) | 452 |
| Staphylococcus aureus (S.aureus) | 87 |
|
Enterobacter aerogenes E.aerogenes) |
111 |
|
Pseudomonas aeruginosa (P.aeruginosa) |
424 |
| Salmonella typhi (S.typhi) | 733 |
|
Staphylococcus epidermidis (S.epidermidis) |
435 |
|
Salmonella typhimurium (S.typhimurium) |
98 |
| Proteus vulgaris (P. vulgaris) | 426 |
Agar disc diffusion antibiotic activities
For antibacterial properties, 0.1 ml bacterial suspension of 105 CFU ml−1 was uniformly spread on Mueller-Hinton Agar plate to form lawn cultures. The aqueous, acetone, ethanol and methanol extracts were prepared in their respective solvents in such a manner that ultimate amount (in dry form) in each disc came to 10mg, 8mg, 6mg, 4mg and 2mg. The blotting paper discs (10mm diameter) were soaked in various diluted extract, dried in oven at 60°C to remove excess of solvent and tested for their antibacterial activity against bacterial pathogens by disc diffusion technique. After incubation of 24 hr at 37°C, zone of inhibition of growth was measured in mm. Ampicillin 10mcg (Hi-Media disc) was used as positive control while discs soaked in various organic solvents and dried were placed on lawns as negative control.
Results and Discussion
During the past decades, traditional systems of medicine have become increasingly important in view of their safety. A current estimate suggests that, in many developing countries, a large proportion of population relies heavily on traditional practitioners and medicinal plants to meet primary health care needs. The present study was conducted to investigate antibacterial properties of fruits of H.isora, which is less studied and used in Indian Folkloric Medicine. Herbal remedies play a fundamental role in traditional medicine in rural areas of India where the therapeutic treatment of choice as antiseptic, anti-inflammatory and in treatment of infectious diseases including diarrhoea. In present study attempt was made to correlate traditional herbal medicinal knowledge held by the Indian native people with modern scientific laboratory-based assay.
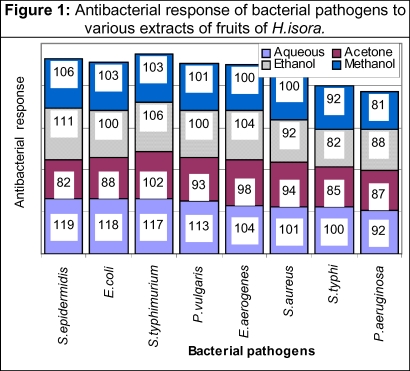
The amount of dry extracts recovered from various solvents is shown in Table 2. Antibacterial activities of aqueous, acetone, ethanol and methanol extracts of fruits of H. isora were studied. The aqueous extracts showed prominent antibacterial activities against E.coli, S.epidermidis and P.vulgaris and S.typhimurium whereas it was moderate against S.aureus, E.aerogenes and S. typhi and least against P. aeruginosa. The acetone extract showed maximum activity against S. typhimurium, moderate against S.aureus, E.aerogenes and P.vulgaris and least against E.coli, P. aeruginosa, S. typhi and S. epidermidis. The ethanol extract showed prominent antimicrobial activities against S.epidermidis, S. typhimurium and E.aerogenes, moderate against E.coli, P.vulgaris, S.aureus and least against S. aureus, P.aeruginosa and S.typhi. The methanol extract showed maximum antibacterial activities against S. epidermidis, E.coli, S. typhimurium and P. vulgaris, moderate against S.aureus, E. aerogenes, and least against S.typhi and P. aeruginosa (Table 3). The aqueous extract showed maximal, the ethanol and methanol extract moderate and acetone extracts least antibacterial activities. The aqueous extract showed maximum antibacterial activities against the tested bacterial pathogens, it might be due to higher solubility of antibacterial principles in water as compared to ethanol, methanol and acetone. . Moreover, the fruits are highly fibrous and difficult to ground in fine powder, which may hinder to extract antibacterial component by organic solvents (Figure 1)
Table 2.
Average recovery of dry extract from 20g of fruit powder
| Type of extract |
Amount (g) |
| Aqueous | 12.56 |
| Acetone | 10.43 |
| Ethanol | 11.26 |
| Methanol | 10.15 |
Table 3.
Antibacterial activity of H. isora fruit extracts against enteric pathogens (Zone of inhibition of growth in mm, average of 5 readings)
| Bacterial pathogens | Aqueous Extract | Acetone extract | Ethanol Extract | Methanol extract | Controls | ||||||||||||||||||||
| 10 mg/disc | 8 mg/disc | 6 mg/disc | 4 mg/disc | 2 mg/disc | 10 mg/disc | 8 mg/disc | 6 mg/disc | 4 mg/disc | 2 mg/disc | 10 mg/disc | 8 mg/disc | 6 mg/disc | 4 mg/disc | 2 mg/disc | 10 mg/disc | 8 mg/disc | 6 mg/disc | 4 mg/disc | 2 mg/disc | Ampicillin (10mcg) |
DW | Acetone | Ethanol | Methanol | |
| E. coli | 30 | 27 | 24 | 20 | 17 | 24 | 19 | 17 | 15 | 13 | 26 | 23 | 20 | 17 | 14 | 27 | 22 | 20 | 19 | 15 | 15 | - | - | 11 | 11 |
| S. aureus | 27 | 23 | 20 | 16 | 14 | 24 | 22 | 18 | 16 | 14 | 24 | 20 | 18 | 16 | 14 | 24 | 22 | 21 | 19 | 14 | 17 | - | 12 | 12 | 0 |
| E. aerogenes | 26 | 24 | 20 | 19 | 15 | 25 | 22 | 19 | 17 | 15 | 26 | 23 | 21 | 18 | 16 | 24 | 23 | 20 | 18 | 15 | 15 | - | 11 | 12 | 11 |
| P.aeruginosa | 22 | 21 | 18 | 17 | 14 | 21 | 19 | 17 | 15 | 13 | 22 | 20 | 17 | 16 | 13 | 20 | 17 | 16 | 14 | 14 | 16 | - | 12 | 0 | 14 |
| S.typhi | 24 | 21 | 20 | 19 | 16 | 21 | 19 | 17 | 15 | 13 | 22 | 20 | 17 | 15 | 13 | 23 | 21 | 18 | 16 | 14 | 18 | - | 13 | 13 | - |
| S. epidermidis | 30 | 26 | 24 | 21 | 18 | 24 | 21 | 19 | 16 | 14 | 26 | 25 | 23 | 20 | 17 | 25 | 23 | 22 | 20 | 16 | 18 | - | - | 13 | - |
|
S. typhimurium |
28 | 26 | 24 | 21 | 18 | 25 | 23 | 21 | 18 | 15 | 26 | 24 | 21 | 19 | 16 | 25 | 24 | 20 | 18 | 16 | 16 | - | 11 | 12 | 11 |
| P. vulgaris | 29 | 25 | 22 | 20 | 17 | 24 | 21 | 19 | 15 | 14 | 25 | 23 | 20 | 17 | 15 | 24 | 22 | 20 | 19 | 16 | 16 | - | 11 | 12 | - |
Figure 1.
Antibacterial response of bacterial pathogens to various extracts of fruits of H.isora.
The fruits of H.isora contain various phytochemical components such as carbohydrates; anthraquinon glycosides, proteins, tannin and phenolic compounds and steroids (Table 4) and combination or joint action of these components in extracts may contribute to the antibacterial properties.
Table 4.
Photochemical analysis of Helicteres isora fruits
| Phytochemicals | Result |
| Alkaloid | Absent |
| Flavonoids | Absent |
| Carbohydrates | Present |
| Cardiac Glycosides | Absent |
| Anthraquinon Glycosides |
Present |
| Saponins | Absent |
| Proteins | Present |
| Tannin and Phenolic compounds |
Present |
| Volatile oils | Absent |
| Steroids | Present |
These fruits are employed in intestinal disturbance such as colic flatulence, diarrhoea, chronic dysentery and stomach-ache (Prajapati et al, 2003). Badgujar et al, (2006) had studied antimicrobial activity of Stem bark of H. isora and showed antimicrobial activity against S. aureus, Bacillus subtilis, P. aeruginosa and E. coli. In the present study, the fruits extracts also showed similar antibacterial activities against these pathogens. Bonjar et al, (2004) showed anti-Candida albicans activity of fruits of H. isora. In the present study, the fruit of H. isora showed antibacterial activity against all tested enteric pathogens. It authenticates the use of fruits of H. isora in the treatment of enteric or diarrhoeal infections and supports the traditional use of the plant.
Impact of study
It is clear that H.isora L plant's fruits used by people against diarrheal disease showed antibacterial activities. Although the nature and number of active antibacterial principles involved in fruit paste of H. isora are not clear in present research, but the broad spectrum activity of fruit paste especially on enteric pathogens, is promising. The present study suggests that fruits of H.isora L are antibacterial against enteric and diarrhoeal bacterial pathogens. The results of present study may form the basis for further investigation to isolate active compounds, elucidate the structure and evaluate them against wider rage of drug-resistant bacterial strains.
References
- 1.Badgujar VB, Jain PS, Pal SC, Patil RR. Antimicrobial activity of stem bark of Helicteres isora. Indian Journal of Natural Products. 2006;22(2):34–35. [Google Scholar]
- 2.Ballal M. Screening of medicinal plants used in rural folk medicine for treatment of diarrhoea. 2005. Internet: http://www.Pharmoinfo.net.
- 3.Bean MF, Antoun M, Abramson D, Chang CJ, Mc Laughlin JL, Cassady JM. Cucurbitacin B and Isocucurbitacin B cytotoxic components of H.isora. J Nat Prod. 1985;48:500–503. doi: 10.1021/np50039a033. [DOI] [PubMed] [Google Scholar]
- 4.Bonjar SGH. Anti yeast activity of some plants used in traditional herbal-medicine of Iran. J Biological Sci. 2004;4(2):212–215. [Google Scholar]
- 5.Chopra EGC, Nayar Sl, Chopra IC. Glossary of Indian Medicinal Plants. 1st Edn. New Delhi, India: CSIR; 1956. p. 131. [Google Scholar]
- 6.Khandelwal K R. Practical Pharmacognosy Techniques and Experiments. 8th edn. Pune: Nirali Publication; 2001. Preliminary phytochemicals screening; pp. 149–156. [Google Scholar]
- 7.Kirtikar KR, Basu BD. Indian Medicinal Plants. Vol. 1. Dehardun, India: International book distributors; 1995. pp. 371–372. [Google Scholar]
- 8.Krishnaraju AV, Rao Tayi V N, Sundararaju1 D, Vanisree Ml, Tsay Hsin-Sheng, Subbaraju1 GV. Biological Screening of Medicinal Plants Collected from Eastern Ghats of India Using Artemia salina (Brine Shrimp Test) Int J Appl Sci Eng. 2006;4(2):115–125. [Google Scholar]
- 9.Kumar G, Murugesan AG, Pandian MRajasekara. Effects of Helicteres isora bark extract on blood glucose and hepatic enzymes in experimental diabetes. Pharmazie. 2006;61:353–355. [PubMed] [Google Scholar]
- 10.Kumar G, Banu GS, Murugesen AG, Pandian MR. Preliminary toxicity and phytochemical studies of aqueous bark extract of Helicteres isora L. Inter J Pharmacol. 2007;3(1):96–100. [Google Scholar]
- 11.Pohocha N, Grampurohit ND. Antispasmodic activity of fruits of Helicteres isora Linn. Phytother Res. 2001;15:49–52. doi: 10.1002/1099-1573(200102)15:1<49::aid-ptr729>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Prajapati ND, Purohit SS, Sharma A, Kumar T. A handbook of medicinal plants. 1st edn. 2003. Medicinal plants-Helicteres isora; p. 18. 302. [Google Scholar]
- 13.Satake T, Kamiya K, Saiki Y, Ham T, Fujimoto Y, Kitanaka S, Kimura Y, Uzawa J, Endang H, Umar M. Studies on the Constituents of Fruits of Helicteres isora L. Chem Pharm Bull. 1999;47(10):1444–1447. [Google Scholar]
- 14.Singh KK, Saha S, Maheshwari JK. Ethnobotany of Helicteres isora Linn. In Kheri district, Uttar Pradesh. J Economic Taxonomic Botany. 1985;7(2):487–492. [Google Scholar]
- 15.World Health Organization, author. Summary of WHO guidelines for the assessment of herbal medicines. Herbal Gram. 1993;28:13–14. [Google Scholar]
- 16.World Health Organization, author. Programme for control of diarrhoeal diseases, Geneva. WHO Bulletin. 1985;63:557–772. 5th Programme Report. [Google Scholar]