Abstract
This study investigated the effect of Catha edulis (khat) on some important parameters of the metabolic syndrome in Wistar Ottawa Karlsburg W (WOKW ) rat. The animals were fed with the standard chow containing 5% air dried pulverized khat leaves for 14 days; followed by the standard chow for16 days. The khat leaves were sorted into green (khat light; KL) and crimson (khat dark; KD) leaves. The control rats were fed on standard chow. Blood glucose (G), serum insulin, serum leptin and serum lipids (triglycerides, total cholesterol, HDL-, LDL-, and VLDL cholesterol) were determined. Feeding with khat leaves reduced the body weight and the triglyceride level of the animals. The effect of KD on these parameters was stronger than that of KL. KD lowered the blood glucose concentration and the leptin content whereas KL was inactive. The khat intake had no significant influence on serum insulin, total serum cholesterol, HDL-, LDL- and VLDL-cholesterol.
Keywords: Catha edulis, WOKW rats, metabolic syndrome
Introduction
Khat is an evergreen shrub which grows at high attitudes. All the plant leaves can be freshly chewed, but the soft small top (crimson) and green leaves are preferred. The chewing of khat (Catha edulis) leaves is widely practiced in East Africa and parts of the Arabian Peninsula. Notably in Yemen, it has a deep-rooted social and cultural tradition. People chew the fresh young leaves for their stimulant and pleasurable effects (Al-Motarreb et al., 2002) which are mainly attributed to cathinone (Kalix, 1990). An enormous amount of data has been published on pharmacology and chemistry of khat, but only few reports are available about its effect on blood glucose and lipids (Al-Zubairi et al., 2003). The present study was carried out to evaluate the effect of Catha edulis on some elements of the metabolic syndrome in Wistar Ottawa Karlsburg W (WOKW) rats. Comparative studies have shown that the Wistar Ottawa Karlsburg W rat strain develops a nearly complete metabolic syndrome with obesity, hyperleptinemia, moderate hypertension, dyslipidemia, hyperinsulinemia and impaired glucose tolerance. Like in human the metabolic syndrome in WOKW is polygenetically inherited. Therefore, the WOKW rat is a suitable model for this study(Klöting et al., 2001, Klöting 2006; Kovacs et al., 2000; van den Brandt et al., 2000, van den Brandt et al., 2002). This pilot study is designed to find out the possible involvement of khat actions in ongoing path of physiological state or complications of metabolic syndrome. Results of this study could be considered as a first step for further studies in animals and human subjects and can apart be helpful in evaluating the pharmacological changes in khat chewers with diabetes or metabolic syndrome.
Since almost all of the investigations of khat effect have been done with whole undifferentiated chewable leaves (smooth crimson and green), the results of such works were often inconsistent. We separated the chewable leaves of the same shrub. After drying, the green leaves became light green (khat light, KL) and the crimson leaves dark green (khat dark, KD).
Methodology
Plant materials
Fresh khat leaves (voucher number 06/2006) harvested in July 2006 from mountain Gehaf (Aldhalea province) were sorted according to colors within the young shrub into small, smooth, crimson (red) and small, smooth, green leaves. The leaves were air died. After drying, the crimson leaves became dark green and the other leaves light green. Then they were pulverized, transported in plastic bags to laboratories of the Institute of Pharmacy, University of Greifswald, Germany, and used within 2 weeks from harvesting. For the purpose of this work, the light green is given the name “khat light” (KL) and the dark green the name “khat dark” (KD).
Experimental Animals and biological investigations
Eighteen-week-old, female WOKW rats bred and kept in the Laboratories of Department of Laboratory Animal Science, Medical Faculty, University of Greifswald, Germany were used. Animals were bred under semi-barrier conditions but the studies were carried out under conventional holding conditions. Animals were divided into 3 groups. The rats in the control group (5 rats) were fed with standard food (Ssniff, Soest, Germany) ad libitum and acidulated tap water. The rats of the experimental groups, group 2 and 3 (6 rats each), were given standard food containing 5% of the air dried pulverized khat light and khat dark, respectively. Food supply was limited to 30 g/day/rat and food intake was determined daily. The feeding with the pulverized leaves continued for 14 days, after that, normal food was given for further 14 days. Body weight gain was determined weekly whereas blood parameters were measured before (day 0); 14 days after khat feeding and 16 days after recovery to normal food (30 days) except for serum leptin, which was only measured at day 0 and 14. Blood was obtained by puncturing of orbital venus plexus to determine blood glucose and serum lipids (triglycerides, total cholesterol, HDL, LDL and VLDL cholesterol). Lipids were measured using the SAS-3 Cholesterol Profile Kit according to the manufacturer's instruction (Helena BioSciences Europe, Sunderland, UK). Serum insulin was determined using the Rat Insulin ELISA-Kit (Mercodia, Uppsala, Sweden) and serum leptin was determined using the Rat Leptin RIA-Kit (DRG Instruments GmbH, Marburg, Germany). To measure glucose tolerance 2 grams glucose per kg body weight were given orally. Blood samples were obtained by tail vein incision at 0 (baseline), 30, 60, 120, and 180 minutes after glucose load. Blood glucose was determined using a glucose analyzer (ESAT 6660-2, Medingen, Germany). All experiments were performed in accordance with the rules for animal care of the Ministry of Nutrition, Agriculture and Forestry of the Government of Mecklenburg-Vorpommern, Germany.
Statistics
Data were given as mean ±SEM. Significant differences were assessed by Student's t-test (comparison of two groups) and by one-way-analysis of variance corrected with Bonferroni-Holm using the analysis system SPSS (SPSS Inc. Chicago, Il, version 12).
Results
Effect of khat on food intake and body weight
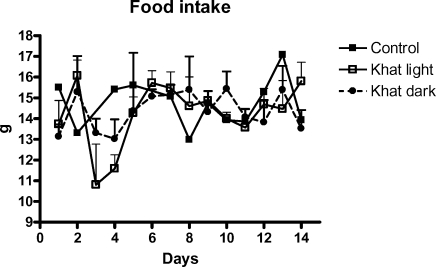
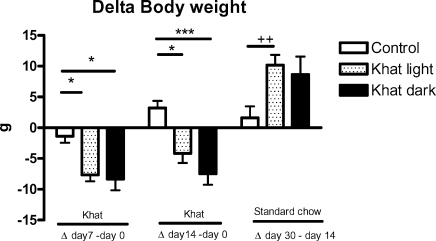
Continued supplementation with Catha edulis leaves 5% of standard food for 14 days did not alter the food intake of the animals (Figure 1). However, their body weight was reduced significantly (Figure 2). Reduction of the weight in rats fed with khat dark, p<0.001, was more than that in those fed with khat light, p<0.05. Interestingly, we observed that after khat application (14 days) and normal feeding with standard food for further 16 days the increase of body weight, shown as delta value of day 30 minus day 14, was 2 times higher than that of the control in case of khat light, p<0.01 (Figure 2).
Figure 1.
Daily intake of food containing 5% khat of both types
Figure 2.
Effect of khat on body weight
Effect of khat on blood glucose
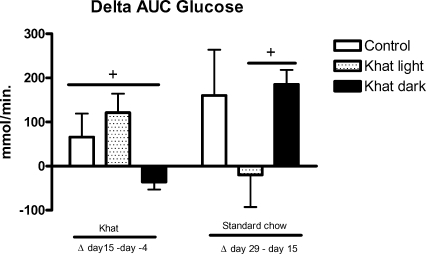
The calculated blood glucose concentrations, area under the curve (AUC), were significantly lower in the group of khat dark in comparison to the control group (p<0.05, Figure 3). Khat light did not influence the AUC significantly. After 16 days standard chow, the khat dark group increased the AUC to the level of control rats, whereas the khat light group did not change their glucose tolerance (Figure 3). The difference between both khat groups is statistically significant (p<0.05).
Figure 3.
Effect of khat on blood glucose
Eighteen-week-old, female WOKW rats were divided into 3 groups. While the rats in the control group were fed with standard food (Ssniff, Soest, Germany) ad libitum and acidulated tap water, group 2 and 3 (each 6 rats), were given standard food containing 5% of the air dried pulverized khat light and khat dark, respectively, for 14 days.
The weight of the body of all rats (control and experimental groups) was measured from day 0 till day 14 during khat intake in case of the experimental groups. Then it was measured for the following days without khat till the 30th day. Body weight was given as . Body weight ± SEM (day 14 – day 0 and day 30 – day 14).
Eighteen-week-old, female WOKW rats were fed with standard food (the control group) or standard food containing 5% of the air dried pulverized khat light and khat dark, respectively for 14 days, were used. Glucose concentrations, area under the curve, AUC (given as Δ values ± SEM) were measured
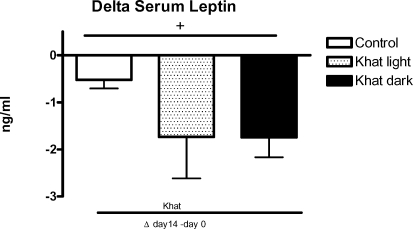
Effect of khat on serum insulin and leptin
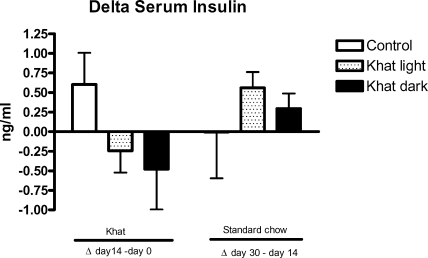
Both leaves decreased insulin (Figure 4) and leptin (Figure 5) release during khat feeding. The serum leptin concentration was significantly lower (p<0.05) in the khat dark group in relation to the control after 14 days of khat feeding (Figure 5). Serum insulin increased slightly, but not significantly, during the feeding period with normal standard chow (Figure 4).
Figure 4.
Effect of khat on serum insulin
Figure 5.
Effect of khat on serum leptin
Female WOKW rats (group 1 and 2) were fed with standard food containing 5% of the air dried pulverized khat light and khat dark, respectively for 14 days. Control rat fed on standard food. The results are given as Δ values ± SEM.
Female WOKW rats (group 1 and 2) were fed with standard food containing 5% of the air dried pulverized khat light and khat dark, respectively for 14 days. Control rat fed on standard food. The results are given as Δ values ± SEM.
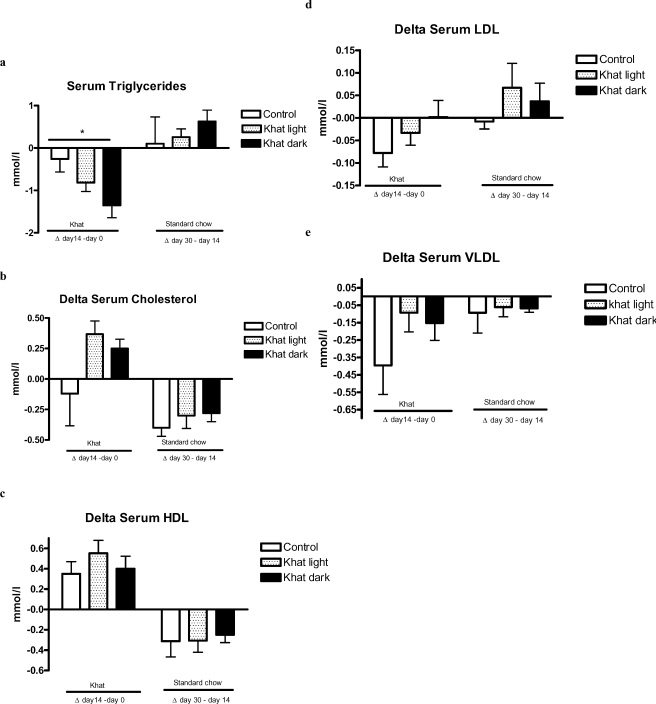
Effect of khat on lipid profiles
Figure 6 (a–e) shows the lipid profiles in rats fed with food containing 5% crude khat. Triglyceride levels (TG) were reduced significantly during khat feeding of both types (p<0.05). After cessation of khat feeding, the TG level increased non-significantly to a level higher than that of the corresponding control. Khat dark reduced the level more than the light did (Figure 6a). The total serum cholesterol and HDL-cholesterol were non-significantly increased (Figure 6 b, c). Similarly, the measured serum LDL- and VLDL-cholesterol levels were only slightly increased during and after the experimentation (Figure 6 d, e).
Figure 6.
Effect of khat on serum lipids
Female WOKW rats (group 1 and 2) were fed with standard food containing 5% of the air dried pulverized khat light and khat dark, respectively for 14 days. Triglyceride levels (TG) (a), total serum cholesterol (b), HDL- (c), LDL (d) - and VLDL-cholesterol (e) level were measured. The results are given as Δ values ± SEM
Discussion
This study was conducted to evaluate the effect of crude Catha edulis in WOKW rats with metabolic syndrome. Reduction of the weight in rats fed with khat is an indication that the plant could be involved in the ongoing metabolic process. Knoll (1979) found and reported that the increase in metabolic rate and oxygen consumption of rats was caused by cathinone. Khat dark slightly disturbed the glucose tolerance as represented by AUC in WOKW rats. However, it seems that the effect in case of KD was short in duration and occurs only during khat intake. Possibly tannins and the inorganic ions present in khat may contribute to the delayed absorption of glucose thereby reducing its level. Moreover, the khat-induced delay of gastric emptying (Heymann et al., 1995) may also play a role in reducing the blood sugar after food intake. The effect of khat chewing in diabetic patients is unclear till now. Some believe that the overall effect of khat in diabetic patients is deleterious, because the user is less likely to follow dietary advice, and the consumption of sweetened beverages with khat raises blood sugar. Investigations in healthy and diabetic khat chewers on blood glucose demonstrated that the level of blood glucose of healthy khat chewers was not affected during khat session because of the sympathetic effect of khat induced release of insulin on the rising blood glucose, while diabetic khat chewers showed non-significant increase in blood glucose at 2 hrs of khat chewing (Saif et al., 2003).
Accelerated gastric emptying increases the food reaching the distal intestine where it stimulates the glucagon like peptide-1 (GLP1)-producing cells and subsequently increases GLP-1 secretion that stimulates beta cells to release insulin (Miholic et al., 1991). Thus, it is possible that khat decreases the gastric emptying rates resulting in less food reaching the distal intestine. Less GLP-1 was produced corresponding to reduced insulin concentrations. In addition, the effect of khat leaves mimics that of amphetamine-like sympathetic action and thus makes it possible to suppress the release of insulin through α-receptor stimulation. It is well proved that leptin, a hormone mainly secreted by white adipocytes (Havel, 2000), provides an important source of information about the size of adipose stores to the central energy regulatory processors in the brain (Pelleymounter et al., 1995). Serum concentrations of leptin are highly correlated with adipose stores (Schwartz, 1996), so reduction in leptin is well correlated with the finding of reduced body weight. Plasma leptin levels are tightly correlated with the total amount of white fat in the body. Leptin has therefore been considered as a “lipostatic factor” contributing to the regulation of body weight via a negative feedback loop (Friedman. and Halaas, 1998). Gettys et al. (1996) reported that hormones like insulin and norepinephrine can modulate leptin transcription and secretion in vivo and in vitro. Administration of norepinephrine or treatment with β-agonists in mice (Rayner and Trayhurn, 2001) decreases plasma leptin levels and leptin transcription in white adipocytes (Moinat et al., 1995). Therefore, it is not surprising that khat or its component cathinone that possesses amphetamine-like sympathetic activity (Kalix, 1992), reduced significantly the level of leptin in our study. In addition, this reduction in leptin may refer to the khat induced suppression of insulin level since insulin stimulates leptin secretion (Cammisotto and Bukowiecki, 2002). At the same time, cathinone, which favours lipolysis mediated through stimulation of β-adrenergic receptors (Kalix, 1992; Kalix and Braenden, 1985), might increase the level of fatty acids that, on the other site, inhibit leptin secretion (Cammisotto et al., 2003). In rabbits, khat leaves reduced significantly the cholesterol, glucose (AUC) and TG concentrations (Al-Habori and Al-Mamary, 2004). In human it was shown that plasma TG, total cholesterol and LDL- cholesterol were non - significantly affected by khat chewers as well as khat chewers who also smoke (Al-Zubairi et al., 2003). Studies in healthy human khat chewers attributed the khat induced reduction in serum TG to the already mentioned amphetamine-like sympathomimetic effects of cathinone (Kalix, 1992; Hassan et al., 2005). The decrease in plasma TG level could also be related to khat-induced reduction in insulin concentration. Since insulin blocks TG degradation, it results in their accumulation. Therefore, a low TG measurement is associated with decrease in insulin level. On the other hand, the increase in the level of VLDL that incorporate TG too could be considered as a result of reduction of insulin concentration that stimulates transcription of lipoprotein lipase and induces VLDL hydrolysis.
Conclusion
Our results clearly show effects of khat on the most important parameters of metabolic syndrome in rats. They need confirmation in human and could be helpful to evaluate the risks and/or potential of khat for human health. Besides it is obvious that there are partly strong differences between the effects of different types of khat leaves (green or light and crimson or dark khat). Our preliminary chromatographic investigations show that the cathinone content in the dark leaves is higher than that in the light leaves (unpublished). Therefore we assume that cathinone contributes to the observed effects. Further phytochemical investigations are necessary to analyse the content of bioactive compounds (cathinone, cathin, tannins and other) in different khat leaves and preparations. Possibly such differences could explain some inconsistent data of khat effects in the literature.
Acknowledgement
The authors like to thank Prof. Dr. Ingrid Klöting and Dr. Barbara Wilke, Laboratory of Animal Scienes, Medical Faculty, University of Greifswald, for help and the permission to perform the experiments in the laboratory, PD Dr. Ulrich Hofmann, Institute of Pharmacology, University of Greifswald, for gas chromatographic analysis.
References
- 1.Al-Habori M, Al-Mamary M. Long-term feeding effects of Catha edulis leaves on blood constituents in animals. Phytomedicine. 2004;11:639–644. doi: 10.1016/j.phymed.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Al-Motarreb A, Baker K, Broadley KJ. Khat: Pharmacological and medical aspects and its social use in Yemen. Phytother Res. 2002;16:403–413. doi: 10.1002/ptr.1106. [DOI] [PubMed] [Google Scholar]
- 3.Al-Zubairi A, Al-Habori M, Al-Geiry A. Effect of Catha edulis (khat) chewing on plasma lipid peroxdation. J Ethnopharmacol. 2003;87:3–9. doi: 10.1016/s0378-8741(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002;283:C244–C250. doi: 10.1152/ajpcell.00033.2002. [DOI] [PubMed] [Google Scholar]
- 5.Cammisotto PG, Gélinas Y, Deshaies Y, Bukowiecki L J. Regulation of leptin secretion from white adipocytes by free fatty acids. Am J Physiol Endocrinol Metab. 2003;285:E521–E526. doi: 10.1152/ajpendo.00052.2003. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 7.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 8.Hassan NA, Gunaid AA, El-Khally FM, Al-Noami MY, Murray-Lyon IM. Khat chewing and arterial blood pressure. A randomized controlled clinical trail of alpha-1 and selective beta-1 adrenoceptor blockade. Saudi Med J. 2005;26(4):537–541. [PubMed] [Google Scholar]
- 9.Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–371. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- 10.Heymann TD, Bhupulan A, Zuriekat NEK, Bomanji J, Drinkwater C, Giles P, Murray-Lyon IM. Khat chewing delays gastric emptying of a semi-solid meal. Aliment Pharmacol Ther. 1995;9:81–83. doi: 10.1111/j.1365-2036.1995.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalix P. Pharmacological properties of the stimulant khat. Pharmacol Ther. 1990;48(3):397–416. doi: 10.1016/0163-7258(90)90057-9. [DOI] [PubMed] [Google Scholar]
- 12.Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol. 1992;70:77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalix P, Braenden O. Pharmacological aspects of the chewing of khat leaves. Pharmacol Rev. 1985;37:149–164. [PubMed] [Google Scholar]
- 14.Klöting I, Kovacs P, van den Brandt J. Sex-specific and sex-independent quantitative trait loci for facets of the metabolic syndrome in WOKW rats. Biochem Biophys Res Commun. 2001;284:150–156. doi: 10.1006/bbrc.2001.4932. [DOI] [PubMed] [Google Scholar]
- 15.Klöting N, Blüher M, Klöting I. The polygenetically inherited metabolic syndrome of WOKW rats is associated with insulin resistance and altered gene expression in adipose tissue. Diabetes Metab Res Rev. 2006;22:146–154. doi: 10.1002/dmrr.582. [DOI] [PubMed] [Google Scholar]
- 16.Knoll J. Problems of Drug Dependence. Vol. 27. Washington, DC: US Government Printing Office; 1979. Studies of the effect of L-cathinone; pp. 322–323. NIDA-Research Monograph. [Google Scholar]
- 17.Kovacs P, van den Brandt J, Klöting I. Genetic dissection of the syndrome X in the rat. Biochem Biophys Res Commun. 2000;269:600–605. doi: 10.1006/bbrc.2000.2352. [DOI] [PubMed] [Google Scholar]
- 18.Miholic J, Orskov C, Holst JJ, Kotzerke J, Meyer HJ. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci. 1991;36:1361–1370. doi: 10.1007/BF01296800. [DOI] [PubMed] [Google Scholar]
- 19.Moinat M, Deng C, Muzzin P, Assimacopoulos-Jeannet F, Seydoux J, Dulloo AG, Giacobino JP. Modulation of obese gene expression in rat brown and white adipose tissues. FEBS Lett. 1995;373:131–134. doi: 10.1016/0014-5793(95)01030-i. [DOI] [PubMed] [Google Scholar]
- 20.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 21.Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- 22.Saif A R, Al-Qirbi A, Al-Geiry A, Al-Habori M. Effect of Catha edulis on plasma glucose and C-peptide in both type 2 diabetics and non-diabetics. J Ethnopharmacol . 2003;86:45–49. doi: 10.1016/s0378-8741(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MW, Peskind E, Raskind M, Boyko E J, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 24.van den Brandt J, Kovacs P, Klöting I. Metabolic features in disease-resistant as well as in hypertensive SHR and newly established obese Wistar Ottawa Karlsburg inbred rats. Int J Obesity. 2000;24:1618–1622. doi: 10.1038/sj.ijo.0801444. [DOI] [PubMed] [Google Scholar]
- 25.van den Brandt J, Kovacs P, Klöting I. Metabolic syndrome and ageing in Wistar Ottawa Karlsburg W rats. Int J Obesity. 2002;26:1–4. doi: 10.1038/sj.ijo.0801966. [DOI] [PubMed] [Google Scholar]