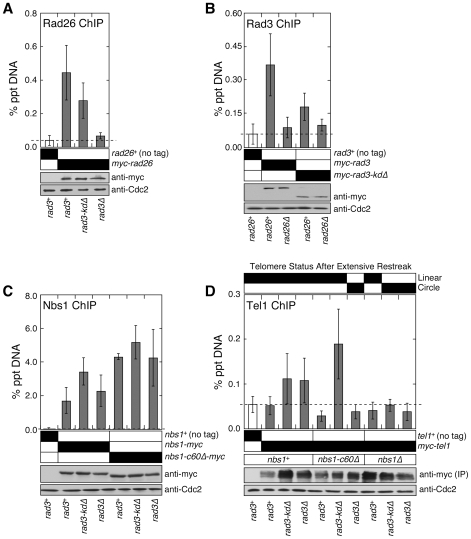
Figure 2. The C-terminal Tel1ATM interaction domain of Nbs1 and the non-kinase domain of Rad3ATR redundantly contribute to recruitment of Tel1ATM to telomeres.
(A) Recruitment of Rad26ATRIP to telomeres in rad3+, rad3-kdΔ, and rad3Δ, monitored by quantitative ChIP assays. Mean values plus or minus one standard deviation for two to nine independent experiments are plotted. Compared to untagged control, Rad26ATRIP showed significant telomere binding in rad3+ and rad3-kdΔ (P values≤0.00003), but not in rad3Δ (P = 0.282). Based on anti-myc western blot, comparable level of Rad26 was expressed. Anti-Cdc2 western blots were used as loading controls. (B) Recruitment of wild-type Rad3ATR or Rad3-kdΔ to telomeres in rad26+ or rad26Δ, monitored by quantitative ChIP assays. Mean values plus or minus one standard deviation for three to eleven independent experiments are plotted. Compared to untagged control, both wild-type Rad3 and Rad3-kdΔ showed significant telomere binding in rad26+ (P values≤0.0003) but not in rad26Δ (P values≥0.154). Based on anti-myc western blot, expression levels of wild-type or truncated Rad3ATR are not affected by deletion of rad26. Anti-Cdc2 western blots were used as loading controls. (C) Recruitment of wild-type Nbs1 or Nbs1-c60Δ to telomeres in rad3+, rad3-kdΔ, or rad3Δ, monitored by quantitative ChIP assays. Mean values plus or minus one standard deviation for three to seven independent experiments are plotted. Compared to untagged control, both wild-type Nbs1 and Nbs1-c60Δ showed significant telomere binding in all genetic backgrounds tested (P values≤0.0001). Telomere binding of wild-type Nbs1 was significantly increased in rad3-kdΔ (P = 0.004) but not rad3Δ cells (P = 0.295), compared to rad3+ cells. For Nbs1-c60Δ, there was no significant change in telomere association in rad3-kdΔ (P = 0.214) or rad3Δ cells (P = 0.952), compared to rad3+ cells. Based on anti-myc western blot, expression levels of wild-type or truncated Nbs1 are not affected by mutations in Rad3. Anti-Cdc2 western blots were used as loading controls. (D) Recruitment of Tel1ATM to telomeres in various mutant combinations among nbs1 and rad3, monitored by quantitative ChIP assays. Mean values plus or minus one standard deviation for two to four independent experiments are plotted. Only rad3-kdΔ, rad3Δ and rad3-kdΔ nbs1-c60Δ showed higher mean % precipitated DNA (% ppt DNA) values compared to untagged control. Tel1 binding to telomeres in rad3-kdΔ and rad3Δ cells was deemed statistically insignificant (P values 0.155 and 0.139, respectively) compared to untagged control, due to large standard deviation in % ppt DNA values among four independent ChIP experiments. However, we consistently found that % ppt DNA values for rad3-kdΔ and rad3Δ cells are higher than untagged control or rad3+ cells for a given set of ChIP experiment performed the same day. Tel1 binding to telomeres in rad3-kdΔ nbs1-c60Δ cells, compared to untagged control, was statistically significant (P = 0.016). Since myc-Tel1 expressed from its endogenous promoter could not be detected in whole cell extracts by anti-myc western blot, anti-myc immunoprecipitation (IP) was performed to enrich for myc-Tel1. Based on anti-myc western blot, comparable amounts of Tel1 were pulled down in different mutant backgrounds. Anti-Cdc2 western blots were used as loading controls.