We read with great interest the systematic review and meta-analysis by Gurion et al. assessing the efficacy of hypomethylating agents (HMA) versus supportive care for the treatment of patients with myelodysplastic syndromes (MDS).1 The meta-analysis included 4 randomized controlled trials (RCT). As the authors noted, we also performed a meta-analysis/systematic review on the same topic that included the same RCTs.2,3 For the benefit of the medical community, it is important to see the reproducibility achieved by two groups working independently. Our meta-analysis conclusions differed slightly and contained additional elements, which in our opinion, further strengthens the report by Gurion and colleagues.1
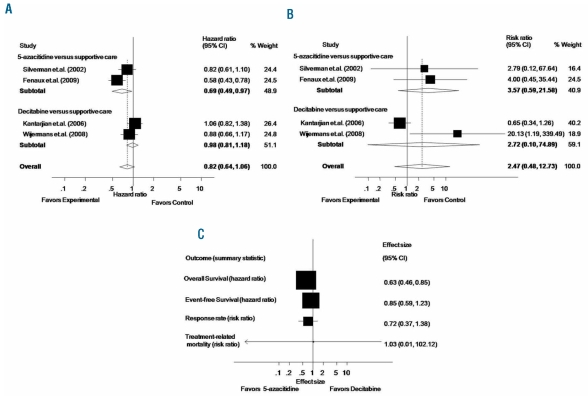
First, Gurion et al.1 did not report survival data from the study by Kantarjian et al.4 We were able to extract survival data from that study using the Parmar method.5 Second, for the study of Silverman et al.,6 we calculated a hazard ratio of 0.82 and corresponding 95% CI of 0.61–1.10, a statistically non-significant difference, while Gurion et al.1 reported a statistically significant difference between 5-azacitidine and supportive care with a hazard ratio of 0.52 along with 95% CI of 0.32–0.86. We believe our data are closer to the original estimates since Silverman et al.6 also reported a statistically non-significant difference in median survival between the 5-azacitidine and supportive care for the intention-to-treat population (P=0.10). The inclusion of survival data from the study by Kantarijan et al.,4 as well as corrected hazard ratio for survival by Silverman et al.6 impacts the results of the meta-analysis. As shown in Figure 1A, pooled results from 4 RCTs enrolling 952 patients show no difference in overall survival for the comparison of HMA versus supportive care (hazard ratio 0.82, 95% CI 0.64–1.06; P=0.124) while Gurion et al. report overall survival benefit with HMA (hazard ratio 0.71, 95% CI 95% CI 0.58–0.87).1 It is important to note, however, that for the comparison of 5-azacitidine versus supportive care the survival benefit still holds (hazard ratio 0.62, 95% CI 0.48–0.78; P=0.030). On the other hand, a survival benefit was not seen for the decitabine versus supportive care. The pooled hazard ratio for overall survival comparing decitabine versus supportive care from 2 trials, instead of one trial reported by Gurion et al.,1 enrolling 403 patients, instead of 233,4,7 is 0.98 and the corresponding 95% CI is 0.81–1.18 (P=0.815).
Figure 1.
Meta-analysis for the outcome of overall survival (A) and treatment-related mortality (B) of 952 patients enrolled in 4 randomized controlled trials. Adjusted indirect meta-analysis of randomized controlled trials on the efficacy of hypo-methylating agents for the treatment of myelodysplastic syndromes (C). The pooled summary effect estimate (hazard ratio/risk ratio) for each study/outcome is indicated by black rectangles, with the lines representing 95% confidence intervals (CIs). The vertical line indicates no difference between two treatments.
We also obtained a somewhat different estimate for treatment-related mortality. Gurion et al.1 pooled 3 trials and the results showed that HMA are associated with a statistically significant risk for treatment-related mortality (risk ratio 7.27, 95% CI 1.67–31.64). As shown in Figure 1B, when all 4 trials are included in the meta-analysis, the risk ratio for treatment-related mortality (4 RCTs, 952 patients) is 2.47 with 95% CI 0.48–12.73 (P=0.281) indicating a statistically non-significant difference with HMA versus supportive care.8–10
Finally, we performed an adjusted indirect meta-analysis to assess the efficacy of 5-azacitidine compared to decitabine using the methods of Bucher,8 Lumley10 and Glenny et al.9 According to this method, an unbiased indirect comparison of interventions of 5-azacitidine versus decitabine can be obtained by adjusting the results of their direct comparisons with a common intervention of supportive care, representing the preferred approach in the absence of a prospective randomized head-to-head study. As shown in Figure 1C, an indirect comparison of 5-azacitidine versus decitabine showed a statistically significant benefit for the outcome of overall survival with 5-azacitidine. The hazard ration for overall survival is 0.63 (95% CI 0.46–0.85; P=0.003). However, there was no difference between 5-azacitidine and decitabine for time to AML transformation or death (HR 0.85; 95% CI 0.59–1.23; P=0.406), response rate (risk ratio 0.716; 95% CI 0.372–1.375; P=0.315, using the number of non-responders) or treatment related mortality (risk ratio 1.026; 95% CI 0.01–102.118; P=0.991).
The results presented here are an important addition to and complement the systematic review by Gurion et al.1 They provide greater precision to the existing results and the supplemental analyses, which in turn will be helpful in making informed decisions on the choice of an optimal HMA for the treatment of MDS in the absence of randomized comparisons.
References
- 1.Gurion R, Vidal L, Gafter-Gvili A, Belnik Y, Yeshurun M, Raanani P, et al. 5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome - systematic review and meta-analysis. Haematologica. 2010;95:343–2. doi: 10.3324/haematol.2009.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, List AF, Mhaskar R, Djulbegovic B. Efficacy of Hypo-Methylating Agents in the Treatment of Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. ASH Annual Meeting Abstracts; 2008 November 16; 2008. p. 3632. [Google Scholar]
- 3.Kumar A, List AF, Mhaskar R, Djulbegovic B, editors. Efficacy of Hypo-Methylating Agents in the Treatment of Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. 14th Congress of the European Society of Hematology; 2008 June 4th to 7th; Berlin. [Google Scholar]
- 4.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer . 2006 Apr 15;106(8:):1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 5.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statist Med. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol . 2002 May 15;20(10:):2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 7.WijerMans P, Suciu S, Baila L, Platzbecker U, Giagounidis A, Selleslag D, et al. Low Dose Decitabine Versus Best Supportive Care in Elderly Patients with Intermediate or High Risk MDS Not Eligible for Intensive Chemotherapy: Final Results of the Randomized Phase III Study (06011) of the EORTC Leukemia and German MDS Study Groups. ASH Annual Meeting Abstracts; 2008 November 16; 2008. p. 226. [DOI] [PubMed] [Google Scholar]
- 8.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of clinical epidemiology. 1997;50(6):683–91. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 9.Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health technology assessment (Winchester, England) 2005;9(26):1–134. iii–iv. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- 10.Lumley T. Network meta-analysis for indirect treatment comparisons. Statistics in medicine. 2002;21(16):2313–24. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]