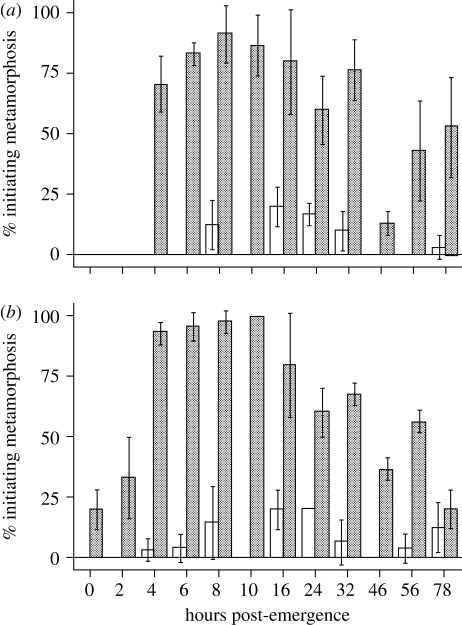
Figure 1.
Acquisition of competence in A. queenslandica larvae. Larvae were either introduced to inductive CCA rubble (stippled bars) or placed in FSW (white bars) at different times after emerging from the adult sponge (x-axis) and the percentage initiating metamorphosis was scored after (a) 4 h and (b) 24 h. Error bars are one standard deviation of the mean. Times post-emergence represent the minimal time emerged over a 2 h window (e.g. 3 h = 3–5 hpe). Methods: A. queenslandica larvae were collected over a period of 2 h from wild adults obtained from the field an hour before initiation of the experiment, and maintained in ambient sea water as described in Leys et al. (2008). All settlement assays were performed at 24°C in six-well 35 mm diameter sterile polycarbonate tissue culture dishes, with 10 ml of FSW per well. For induction experiments, six sets (replicates) of 10 larvae were incubated continuously in wells containing either 10 ml FSW or 10 ml FSW with CCA shards covering approximately 25% of the bottom of the well. CCA shards were chipped off the surfaces of coral rubble pieces collected from the same habitat as A. queenslandica just prior to experimentation, washed and placed in the wells for immediate use. We assayed different aged swimming larvae, spanning 0–78 hpe from the adult. Experiments used larvae that were collected over a 2 h period. For example, larvae termed 0 hpe are from a pool of individuals that together represent 0–2 hpe. After the addition of larvae to both control wells and CCA rubble-containing wells, dishes were incubated in the dark for either 4 or 24 h. Separate cohorts of larvae were used to score rates of metamorphosis at each of these times. At the conclusion of the experiment, each well was scanned to record the number of larvae that had initiated metamorphosis. This was defined by the larval anterior being clearly attached to the substratum, the primary larval axis (anterior–posterior axis) being compressed and the larval pigment ring being resorbed (Leys & Degnan 2002).