Abstract
Little is known about how best to deploy scarce resources for disease control when epidemics occur in different but interconnected regions. We use a combination of optimal control methods and epidemiological theory for metapopulations to address this problem. We consider what strategy should be used if the objective is to minimize the discounted number of infected individuals during the course of an epidemic. We show, for a system with two interconnected regions and an epidemic in which infected individuals recover and can be reinfected, that equalizing infection in the two regions is the worst possible strategy in minimizing the total level of infection. Treatment should instead be preferentially directed at the region with the lower level of infection, treating the other subpopulation only when there is resource left over. The same strategy holds with preferential treatments of regions with lower levels of infection when quarantine is introduced.
Keywords: epidemiological modelling, economic modelling, quarantine, spatio-temporal epidemics, control theory
1. Introduction
Many epidemics outstrip the resources available to treat all infected individuals (Lipsitch et al. 2000), especially when disease occurs simultaneously in different but interconnected regions (Ferguson et al. 2001; Keeling et al. 2001; Dye & Gay 2003). Seeking to control in more than one region poses a dilemma for epidemiologists and health administrators of how best to deploy limited resources among different regions: should preference be given to treating infected individuals in regions with high or with low levels of infection, or to equalizing levels of infection in different regions as fast as possible? Choosing between these options requires a combination of epidemiological and economic insights that hitherto have tended to remain separate: epidemiological models take little account of economic constraints (Forster & Gilligan 2007; Klein et al. 2007), while economic models mostly ignore the spatial and temporal dynamics of disease (Gilligan 2003), with some recent exceptions (Goldman & Lightwood 2002; Rowthorn & Brown 2003; Smith et al. 2005; Barrett & Hoel 2007; Forster & Gilligan 2007), of which Smith et al. (2005) and Forster & Gilligan (2007) explicitly consider infection space.
The influence of the spatial structure of susceptible populations on the invasion and persistence of human, animal and plant pathogens is now well established (Ferguson et al. 2001; Keeling et al. 2001; Dye & Gay 2003; Stacey et al. 2004). Most contemporary epidemiological theories are focused on the dynamics of disease in so-called ‘structured metapopulations’ (Gyllenberg et al. 1997; Grenfell & Bolker 1998; Keeling & Gilligan 2000a; Hanski & Ovaskainen 2002) following on from early models that addressed spatial heterogeneity in disease transmission (Lajmanovich & Yorke 1976; Murray & Cliff 1977; Nold 1980) in which epidemics occur in loosely coupled subpopulations. These subpopulations correspond with natural aggregations of susceptibles, such as hospitals, towns, cities or countries. Infecteds and susceptibles mix more or less freely within subpopulations, with a smaller movement of infecteds or inoculum among subpopulations. The system of loose coupling leads to spatially distributed epidemics with local fade-out but global persistence (Keeling & Gilligan 2000b), as infection is transmitted between infected and healthy subpopulations. It follows that local deployment of control in one region may benefit other regions by reducing the number of infecteds capable of transmitting infection between subpopulations, but the regional benefits of control may also be countermanded by reinvasion from neighbouring populations. Using a combination of optimization methods from the economic theory of disease control (Sethi 1978; Goldman & Lightwood 2002; Rowthorn & Brown 2003; Forster & Gilligan 2007) with a metapopulation model from epidemiological theory (Hanski 1998; Swinton et al. 1998; Park et al. 2003; Keeling et al. 2004), we show, however, that it is possible to optimize the deployment of control. By formalizing the problem as one of control of a dynamic, spatially structured system subject to economic constraints, it becomes apparent that one plausible intuition to give preference to the most highly infected regions when resources are limited may be the worst possible strategy in limiting the amount of infection suffered by the entire population.
2. Methods
2.1. The model
We consider two coupled subpopulations of susceptible individuals, in which an epidemic is described by a simple susceptible–infected–susceptible (SIS) compartmental model. An SIS model is characteristic of a sexually transmitted disease, such as gonorrhoea, in which infecteds (I) recover naturally or after treatment (Lajmanovich & Yorke 1976; Hethcote 1980; Anderson & May 1991). Infected individuals do not gain immunity to the disease, rejoining the susceptible class (S) and so may be reinfected. This relatively simple model of an epidemic allows a rigorous analysis of strategies for optimal control of disease. Here, we consider a simple control strategy in which a certain drug is administered to some or all of the infected individuals in two regions, each with populations of the same size N. The model is inspired by the analysis of Goldman & Lightwood (2002) for optimal drug use in a single region. We envisage regions as encompassing local districts, counties, provinces or countries. The dynamics of infection for the SIS model in the two regions Ii are given by
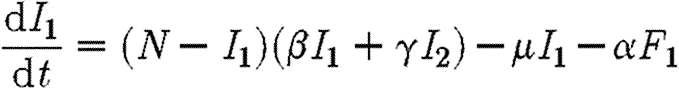 |
2.1 |
and
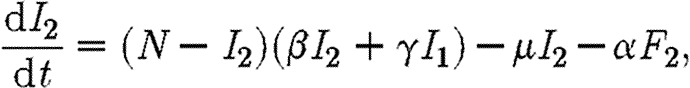 |
2.2 |
in which β and γ are the transmission rates within and between subpopulations, respectively; μ−1 is the infectious period; and α is a measure of the rate at which infecteds are cured by the drug. The number of infecteds receiving treatment in region i is equal to Fi. We assume that the drug is not used as a prophylactic so that only infected individuals receive it, hence Fi≤Ii. An alternative scenario, in which treatment is effected through changes in the transmission rate (β), is discussed briefly in the electronic supplementary material.
2.2. Optimal control under a budget constraint
Suppose that expenditure on drugs is subject to a budget constraint c(F1+F2)≤M. We assume that finance is not transferable through time, so that money which is not spent immediately cannot be saved for the future purchase of drugs. If there are sufficient resources, every infected individual will be treated. Otherwise, drugs are allocated so as to minimize the discounted sum of total infection in the two regions. Hence, we choose F1 and F2 so as to minimize the following integral:
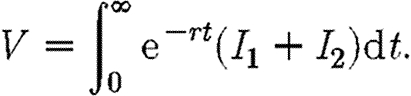 |
2.3 |
The objective function in equation (2.3) is concerned only with minimizing total infection across both subpopulations. We also briefly consider objective functions of the form 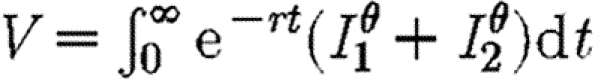 . If θ>1, such an objective function will give extra weight to the area with the higher level of infection. The discount rate is included to allow for long-term changes, thus giving greater emphasis to control in the short rather than the long term (Forster & Gilligan 2007). The optimization approach we adopt is based upon the Hamiltonian method (Pontryagin et al. 1962; Seierstad & Sydsaeter 1987; Pinch 1993), which is a device for minimizing the objective function subject to the economic constraints and the epidemiological dynamics of the model. This method takes into account the influence of current infection on the future evolution of disease as given by the propagation equations (2.1) and (2.2). Such influence is embodied in the co-state variables that appear in a mathematical expression known as the Hamiltonian (see appendix A and the electronic supplementary material for details).
. If θ>1, such an objective function will give extra weight to the area with the higher level of infection. The discount rate is included to allow for long-term changes, thus giving greater emphasis to control in the short rather than the long term (Forster & Gilligan 2007). The optimization approach we adopt is based upon the Hamiltonian method (Pontryagin et al. 1962; Seierstad & Sydsaeter 1987; Pinch 1993), which is a device for minimizing the objective function subject to the economic constraints and the epidemiological dynamics of the model. This method takes into account the influence of current infection on the future evolution of disease as given by the propagation equations (2.1) and (2.2). Such influence is embodied in the co-state variables that appear in a mathematical expression known as the Hamiltonian (see appendix A and the electronic supplementary material for details).
We consider two hypotheses for optimizing the control of infection under a limited budget, when at least for some of the time, the combined number of infected individuals in the two regions exceeds the availability of the drug for treatment. A choice must then be made as to how to optimize the distribution of the drug between the two regions. Under the first hypothesis, preference is given to treating individuals in the region with the higher prevalence, so as to first equalize infection in each region. In the second hypothesis, we assume that preference is given to treating individuals in the region with the lower level of infection, thus seeking to reduce the force of infection in the more sparsely infested region.
2.3. Optimal control under a fixed budget constraint with quarantine
Suppose that the coupling parameter between regions, γ, can be altered by imposing quarantine controls that restrict the reciprocal rate of cross infection between the two regions. These are equivalent to border controls. They may be costly to administer and may also impose indirect costs arising from restrictions on free circulation. Let Q be the total amount of direct and indirect costs involved in the quarantine policy. We shall assume that γ and Q are functionally related as
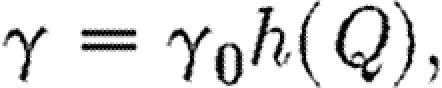 |
2.4 |
where 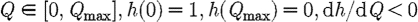 and
and 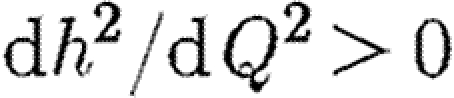 . Thus, when there are no restrictions the cross-infection parameter γ is equal to γ0. When a total ban is imposed to movement of infection between regions γ=0, and the cost of restrictions is equal to Qmax. We also assume that the budgets for medical treatment and for quarantine restrictions are separate, so that funds cannot be transferred between uses. The optimal strategy is now to choose F1, F2 and Q so as to minimize the following integral:
. Thus, when there are no restrictions the cross-infection parameter γ is equal to γ0. When a total ban is imposed to movement of infection between regions γ=0, and the cost of restrictions is equal to Qmax. We also assume that the budgets for medical treatment and for quarantine restrictions are separate, so that funds cannot be transferred between uses. The optimal strategy is now to choose F1, F2 and Q so as to minimize the following integral:
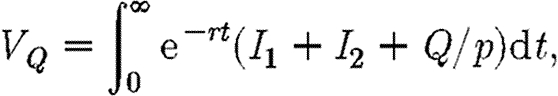 |
2.5 |
subject to the same constraints as before plus the additional constraint 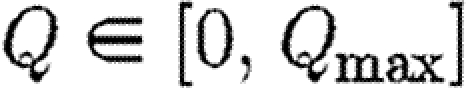 and
and 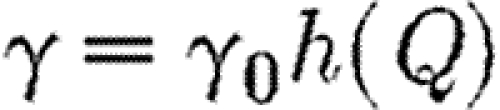 . In this integral, Q/p measures the cost of restrictions expressed in terms of infection equivalents. The methods for optimization are given in appendix A.
. In this integral, Q/p measures the cost of restrictions expressed in terms of infection equivalents. The methods for optimization are given in appendix A.
3. Results
3.1. Preferential treatment of region with higher prevalence
First, we consider preferential treatment of the region with higher prevalence. So long as 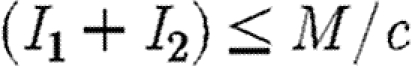 all infected individuals are treated and the infection is either eliminated in each subpopulation, if
all infected individuals are treated and the infection is either eliminated in each subpopulation, if 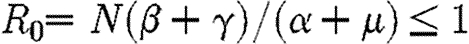 , or brought to some non-negative equilibrium density in each subpopulation if R0>1. When, however, (I1+I2)>M/c, some infecteds remain untreated and a decision must be made as to how to allocate the drug between regions so as to minimize the discounted numbers of infected individuals. One obvious strategy is to equalize the levels of infection within the two regions as fast as possible. Many people would regard this as the socially equitable strategy (Murray & Lopez 1996). Formally, it involves deflection of the two subpopulations onto a spatially homogeneous solution in which the levels of infection within each region are equalized (see appendix A and §S1 in the electronic supplementary material for details). This is conventionally known in optimal control theory as the singular solution (Seierstad & Sydsaeter 1987). Such a strategy would be achieved by preferential treatment of infecteds in the region with the higher prevalence of infecteds. The policy is called the MRAP since it involves the most rapid approach path to the singular solution, in which infection is equalized in both subpopulations (figure 1). However, as we show analytically in appendix A, the MRAP is the worst possible strategy within the constraints of the system. Rather than minimizing the discounted amount of infection over time (equation (2.3)) it maximizes that quantity (figure 1).
, or brought to some non-negative equilibrium density in each subpopulation if R0>1. When, however, (I1+I2)>M/c, some infecteds remain untreated and a decision must be made as to how to allocate the drug between regions so as to minimize the discounted numbers of infected individuals. One obvious strategy is to equalize the levels of infection within the two regions as fast as possible. Many people would regard this as the socially equitable strategy (Murray & Lopez 1996). Formally, it involves deflection of the two subpopulations onto a spatially homogeneous solution in which the levels of infection within each region are equalized (see appendix A and §S1 in the electronic supplementary material for details). This is conventionally known in optimal control theory as the singular solution (Seierstad & Sydsaeter 1987). Such a strategy would be achieved by preferential treatment of infecteds in the region with the higher prevalence of infecteds. The policy is called the MRAP since it involves the most rapid approach path to the singular solution, in which infection is equalized in both subpopulations (figure 1). However, as we show analytically in appendix A, the MRAP is the worst possible strategy within the constraints of the system. Rather than minimizing the discounted amount of infection over time (equation (2.3)) it maximizes that quantity (figure 1).
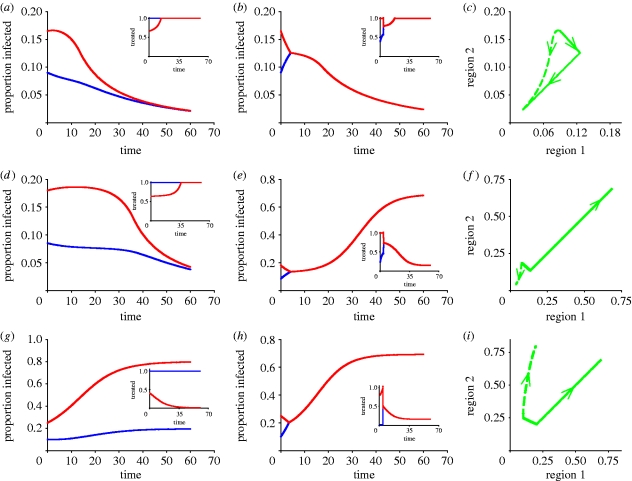
Figure 1.
Comparison of disease progress curves for the (a,d,g) best and (b,e,h) worst policies. (a–c) Progress of disease in two interconnected regions 1 (blue curves) and 2 (red curves), with treatment dynamics in insets, showing small differences between the best and worst paths when initial infection occurs in zone A (table 1). (d–f) The best and worst paths diverge markedly when initial infection occurs in the instability zone B (table 1). (g–i) Disease continues to increase but markedly less steeply in the region with the lower infestation (region 1) when infection occurs in zone C (table 1). Default parameters are α=0.25 (efficiency of control), β=0.25 (within-region transmission rate), γ=0.03 (between-region transmission rate), r=0.1 (discount rate), μ=0.05 (recovery rate), M=0.2 (fixed costs) with N=1 ((c,f,i) proportion infected).
3.2. Finding the optimal strategy
To find the best (i.e. optimal) path, we chose to give preference to the region with the lower level of infection (and, by corollary, the higher level of susceptibles), treating the other region as a residual claimant. Sufficient details of the analysis are given in appendix A to reproduce the results, with additional rigorous mathematical details in the electronic supplementary material. Individuals in the region with the higher level of infection only receive treatment when there are resources left over after treating all the infecteds in the target region (figure 1). Although it is not possible to prove analytically that this is the optimal path, extensive numerical simulations using a variety of parameters support the hypothesis (see appendix A for details). This alternative policy is known as the anti-MRAP since it goes away from the singular solution as fast as possible (figure 1c). By concentrating scarce resources on the least infected region, where there are most susceptibles, we maximize the social benefit associated with the prevention of disease.
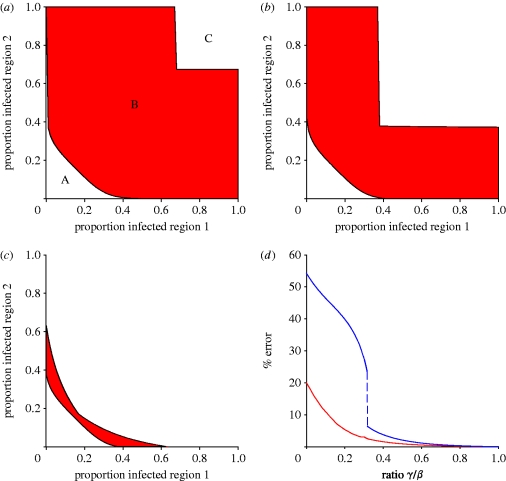
The difference between the best and worst paths depends upon the amount of initial infection in each population when the treatment is first introduced and can be separated into three regimes in infection space (figures 1 and 2; table 1). In zone A, the best and worst scenarios each bring the epidemic under control. The difference between the two is relatively small. In zone B, the worst path fails to bring the epidemic under control while the best path does (figure 1) and the error of choosing the wrong strategy is large (table 1). We refer to this zone as an ‘instability zone’ to indicate the fact that the outcome is highly sensitive to the choice of policy. Neither policy is capable of bringing the epidemic under control in zone C but preferential treatment of the less infected subpopulation is substantially more successful in reducing the discounted amount of infection (figure 1; table 1). The same is also true in the case of the objective function involving 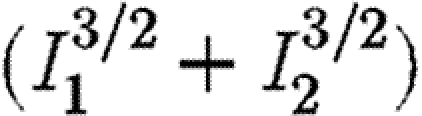 shown in table 1. Thus, even if some weight is assigned to equalizing the levels of infection in the two regions (i.e. with exponents greater than unity), our results show that it may still be better to give priority to the region with the lower level of infection. For completeness, we also show consistency in the results for an objective function involving
shown in table 1. Thus, even if some weight is assigned to equalizing the levels of infection in the two regions (i.e. with exponents greater than unity), our results show that it may still be better to give priority to the region with the lower level of infection. For completeness, we also show consistency in the results for an objective function involving 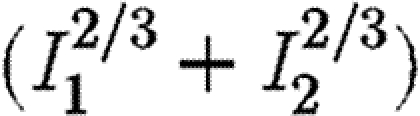 , in which the exponents are less than unity (table 1), implying some penalty for control effort as the level of infection increases.
, in which the exponents are less than unity (table 1), implying some penalty for control effort as the level of infection increases.
Figure 2.
Transmission between regions and the error between the best and worst policies. (a–c) Effect of the between-region transmission rate γ (scaled by β) on the magnitude of the instability zone B (shown in red) in which the best and worst paths lead to marked differences in epidemic behaviour with one controlling the epidemic and the other leading to ‘explosive’ infection towards high levels of infection (figure 1; instability zones for (a) γ/β=0, (b) γ/β=0.008 and (c) γ/β=0.02). (d) Effect of changing the ratio of γ/β on the maximum (blue line) and average (red line) errors between the best and worst policies.
Table 1.
Differences and errors associated with the best and worst strategiesa to control disease in a metapopulation when resources are limited. (Alternative objective functions are shown for the SIS model without quarantine.)
| zone | path | I1(0) | I2(0) | I1(∞) | I2(∞) |
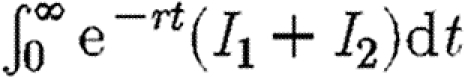 (% error)b (% error)b
|
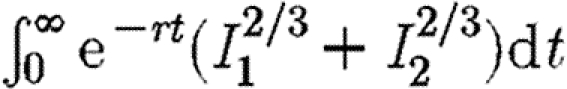 (% error)b (% error)b
|
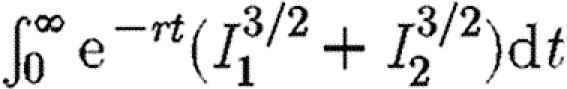 (% error)b (% error)b
|
|---|---|---|---|---|---|---|---|---|
| SIS model without quarantine | ||||||||
| A | worst | 0.090 | 0.165 | 0 | 0 | 2.27 (5.09) | 4.66 (4.59) | 0.78 (3.77) |
| best | 0.090 | 0.165 | 0 | 0 | 2.16 | 4.45 | 0.75 | |
| B | worst | 0.085 | 0.180 | 0.69 | 0.69 | 3.38 (30.05) | 5.99 (19.07) | 1.51 (52.04) |
| best | 0.085 | 0.180 | 0 | 0 | 2.59 | 5.03 | 0.99 | |
| C | worst | 0.100 | 0.250 | 0.69 | 0.69 | 6.14 (18.76) | 8.89 (14.05) | 3.68 (19.81) |
| best | 0.100 | 0.250 | 0.19 | 0.80 | 5.17 | 7.73 | 3.07 | |
| SIS model with quarantine | ||||||||
| C | best | 0.100 | 0.250 | 0 | 0 | 3.83 | ||
1Parameter values as in figure 1.
2Computed by (Vworst−Vbest)/Vbest in which Vworst and Vbest are the values of the discounted infection along the worst and best paths, respectively (see appendix A for details).
3.3. Effects of relative transmission parameters on best and worst solutions
The size of the instability zone B, in which the best and worst paths diverge, depends upon the relative magnitudes of transmission within (β) and between (γ) subpopulations (figure 2). Increasing the value of γ has two effects. It shifts the instability zone inwards reflecting the fact that it has become more difficult to control infection. At the same time, the size of the instability zone shrinks (figure 2). Both the average and maximum values of the error ratio for the difference between the best and worst paths decline as (γ/β) gets larger (figure 2). Thus, as the rate of transmission between subpopulations increases the outcome becomes less sensitive to the choice of policy. Moreover, at a certain point there is a sharp decline in the maximum error ratio. This occurs when γ/β becomes so large that, irrespective of the starting point, it is impossible to contain infection. Under these conditions, zones A and B disappear and zone C covers the entire infection space.
3.4. Quarantine control
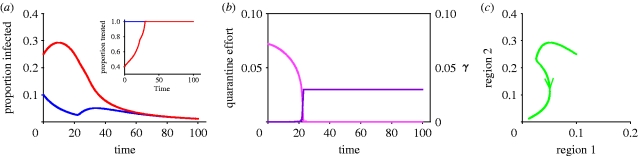
Using the standard procedure (see appendix A) for the propagation equations and the objective function for quarantine introduced in equation (2.5), we derive an optimal value first for 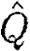 from which it is possible to calculate the corresponding value for quarantine (from equation (2.4)). Extensive numerical analysis again shows that the optimal strategy is the anti-MRAP, giving preference to the population with the lower prevalence of infection while also imposing quarantine to restrict transmission between the two subpopulations (figure 3a). For the example shown in figure 3, a severe quarantine, with γ close to zero (figure 3b), is initially imposed to isolate the high infection region 2. The limited medical resources available are mostly used to saturate the low infection region 1 leaving only a small residual for use in region 2. As infection falls in region 1, more medical resources become available for use in region 2 and infection is eventually brought down there as well. At a certain point, infection is sufficiently low in both regions that it is optimal to lift the quarantine and allow γ to return rapidly to its unrestricted value of 0.03. This is done quite rapidly. Without imposing a temporary quarantine, it is impossible to bring infection down from the starting point shown. With γ fixed at 0.03, total infection increases no matter what treatment policy is followed (cf. figure 1d). Thus, the possibility of quarantine may radically alter the time paths of infection in the two regions. Table 1 compares the integrals for the discounted cost of infection with and without quarantine costs.
from which it is possible to calculate the corresponding value for quarantine (from equation (2.4)). Extensive numerical analysis again shows that the optimal strategy is the anti-MRAP, giving preference to the population with the lower prevalence of infection while also imposing quarantine to restrict transmission between the two subpopulations (figure 3a). For the example shown in figure 3, a severe quarantine, with γ close to zero (figure 3b), is initially imposed to isolate the high infection region 2. The limited medical resources available are mostly used to saturate the low infection region 1 leaving only a small residual for use in region 2. As infection falls in region 1, more medical resources become available for use in region 2 and infection is eventually brought down there as well. At a certain point, infection is sufficiently low in both regions that it is optimal to lift the quarantine and allow γ to return rapidly to its unrestricted value of 0.03. This is done quite rapidly. Without imposing a temporary quarantine, it is impossible to bring infection down from the starting point shown. With γ fixed at 0.03, total infection increases no matter what treatment policy is followed (cf. figure 1d). Thus, the possibility of quarantine may radically alter the time paths of infection in the two regions. Table 1 compares the integrals for the discounted cost of infection with and without quarantine costs.
Figure 3.
The role of quarantine. (a) Disease progress curves in the two regions with treatment allocation shown in the inset, together with quarantine (best path with quarantine; blue line, region 1; red line, region 2). (b) Quarantine effort and corresponding value for γ: note the sudden change in quarantine policy (best path: quarantine effort; light pink line, Q; dark pink line, γ). (c) Phase portrait showing how a potentially explosive epidemic (cf. figure 1j and table 1) can be brought under control by optimizing quarantine together with preferential treatment of the region with the lower prevalence of infection (proportion infected). Default parameters as in figure 1, except that p=1, b=100, Qmax=5 and γ=γ0h(Q), where γ0=0.03 and 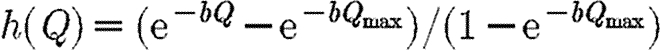 .
.
4. Conclusions and discussion
Epidemics of the SIS form apply to a small but important class of epidemics in which infected individuals recover and can be reinfected (Murray 2002). With just two interconnected regions of hosts and a fixed population size, our SIS formulation allows a rigorous analysis to show that, under certain initial conditions, equalizing infection in each subpopulation is the worst possible strategy when resources are limited for control. It also shows that treatment should be focused on subpopulations with the lower level of infected individuals. This is equivalent to allocating treatment preferentially to subpopulations with the higher proportions of susceptible individuals. The methods introduced here provide new insights into optimal disease control. The results overturn a simple intuition that preference should normally be given to strategies designed to equalize infection in different subpopulations. This paper presents an alternative intuition that derives from the influence of treatment on the future dynamics of disease. Our optimal solution (anti-MRAP) assumes that the benefit to those who are cured is the same no matter where they are located, but invokes the additional policy consideration that curing individuals influences the future dynamics of infection in the coupled subpopulations. The latter does depend upon the location of the individuals who are cured. The most effective way of slowing down the spread of disease is to concentrate on resources in areas where there is a large pool of susceptibles. This implies giving priority to areas with low levels of infection.
Deviation from the optimal strategy necessarily changes this criterion, with greater weight being placed upon the health of some individuals compared with others. That is, preferential treatment of the subpopulation with higher levels of infection and fewer susceptibles necessarily places a greater weighting on the health of individuals in that subpopulation. Such considerations require further debate and greater integration of epidemiological models with insight from social sciences.
One of the principal epidemiological and mathematical challenges is to extend the methods developed here to other realistic, spatially extended epidemics. It is straightforward to extend the analysis to the case of multiple regions and also to the case when treatment reduces the transmission rate of infection, as we point out with illustrative results in the electronic supplementary material (§§S3 and S4, respectively). We conclude from these explorations that the main intuition concerning the inferiority of the MRAP is robust to extensions of the model as listed above. It is our intention to extend these analytical methods further to more complex situations, in which there are immune classes and options for vaccination. In particular, is it the case that the optimal strategy identified in this paper has a counterpart for other classes of epidemics in which treated individuals become immune (SIR) or rejoin the susceptible class (SIRS)? Rigorous mathematical analysis is extremely difficult for these types of epidemics because of the increased number of state and co-state variables involved. Preliminary numerical explorations of an SIR model with births of susceptibles suggest that, for certain initial conditions, it is more efficient to follow the anti-MRAP strategy of giving priority to the infected area with the lower level of infection than it is to equalize infection as fast as possible (see §S5 in the electronic supplementary material). However, further exploratory work suggests that, while giving priority to the region with the greater proportion of susceptibles (analogous to the anti-MRAP strategy in the SIS model) is key to controlling SIR epidemics, the optimal solution sometimes involves switching priority from one subpopulation to another depending upon the initial conditions. This will be the subject of a separate study.
Consideration of the time horizon for control is also important. Here, we have used the conventional economic device of a discount rate to give more weight in the objective function to shorter rather than long-term control. We have used a default value of r=0.1 for the discount rate. Our main result, however, does not depend upon this particular value. The choice of discount rate affects the relative valuation of current and future disease, and also the optimal timing of quarantine: it does not affect the key result that the MRAP is the worst possible strategy.
Forster & Gilligan (2007) analysed an SIS seasonal plant epidemic with transmission of infection between nearest neighbours, approximated by a contact process. Switching the time horizon from a single season (with no discount rate and a fixed time) to multiple seasons (with discount and infinite horizon) effectively changed the optimal solution from a simple bang-bang (treat all then treat none) to a continuously dynamic (interior) response. Interior solutions have also been identified by Sethi (1974) and Goldman & Lightwood (2002) for SIS models, and by Barrett (2003), working with a static epidemic model with bifurcations and a Nash equilibrium, but none of these models addressed the spatial structure of host populations that are shown here to play an important part in the optimization of control. More recently, Barrett & Hoel (2007) have identified criteria under which long-term eradication of diseases, such as poliomyelitis, would be optimal. Using a non-spatial, dynamical SIR model with vaccination of susceptibles, they showed that high rates of vaccination are never optimal. The results contrast with a naive intuition that a high rate of vaccination might be advantageous in eradication programmes in an analogous way to the counter-intuitive results presented here that preference should be given to treating the subpopulation with lower rather than higher levels of infection. All three analyses (Barrett & Hoel 2007; Forster & Gilligan 2007; and the current one) support the insight that may be gained by coupling dynamic epidemiological models with economic optimization.
Acknowledgements
C.A.G. acknowledges support from the Biotechnology and Biological Sciences Research Council.
Appendix A.
The analytical and numerical methods used to derive the results are summarized here in sufficient detail to reproduce the results. Further mathematical detail is given in the electronic supplementary material together with some results for exploratory analysis of an SIR model.
A.1. Optimization methods
The objective is to minimize the discounted level of infection (equation (2.3)) subject to the propagation equations (2.1) and (2.2) (Pinch 1993) and subject to the following epidemiological and economic constraints: Ii(0)=Ii0; 0≤Fi≤Ii, F1+F2=min(I1+I2, M/c). Let 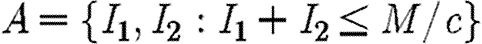 be the region where there are sufficient resources to treat all infecteds. Within this region, the propagation equations are
be the region where there are sufficient resources to treat all infecteds. Within this region, the propagation equations are
| A1 |
These equations have one stable equilibrium, which is given by
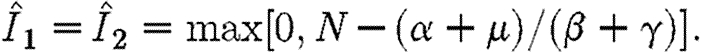 |
A2 |
We assume that 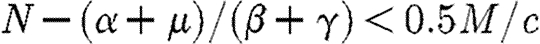 . This ensures that
. This ensures that 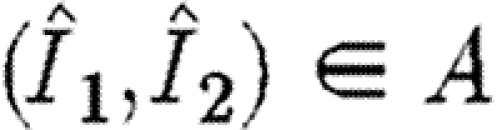 . It also ensures that any allowable path that enters the region A will remain permanently within this region and eventually converge to the stable equilibrium point.
. It also ensures that any allowable path that enters the region A will remain permanently within this region and eventually converge to the stable equilibrium point.
When there are more infecteds than can be treated, c(I1+I2)>M and hence F1+F2=M/c. The relevant Hamiltonian in this case is
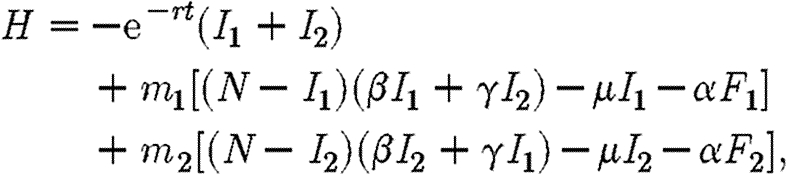 |
A3 |
where mi are co-state variables. Since F2=M/c−F1, we can eliminate F2 to obtain
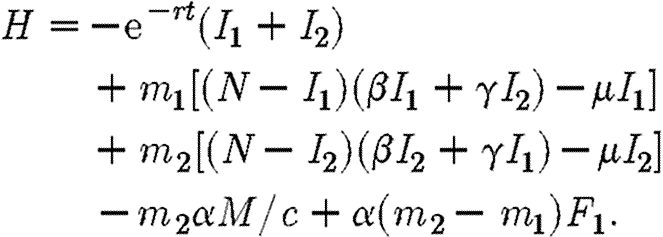 |
A4 |
When c(I1+I2)>M, the control variable F1 is subject to the following inequalities:
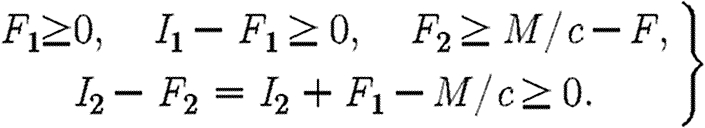 |
A5 |
Some of these are ‘mixed’ constraints, which include both state and co-state variables. In this case, the standard procedure is to include all constraints in a Lagrangian known as the ‘augmented’ Hamiltonian, which is given as follows (Seierstad & Sydsaeter 1987):
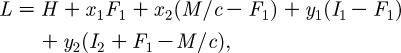 |
A6 |
where the x's and y's are multipliers that satisfy the complementary slack conditions,
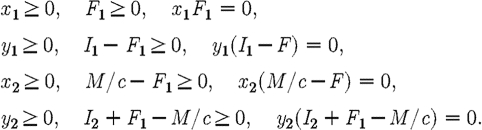 |
A7 |
The first-order conditions for an optimum require that
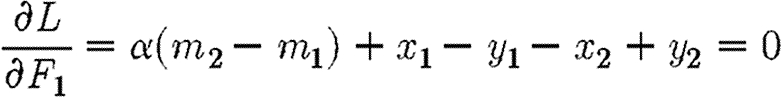 |
A8 |
and that F1 (and hence F2) is chosen so as to maximize the Hamiltonian. This yields the following result:
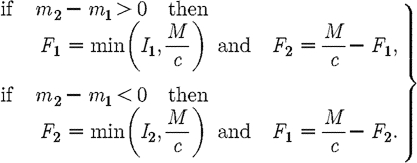 |
A9 |
It must also be the case that
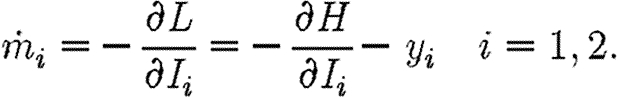 |
A10 |
Finally, there are the transversality conditions. Allowable paths fall into two groups: those that never enter the region 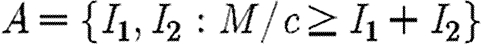 , and those that enter this region and never leave it again. In the former case, there are alternative transversality conditions. Define the function W as follows:
, and those that enter this region and never leave it again. In the former case, there are alternative transversality conditions. Define the function W as follows:
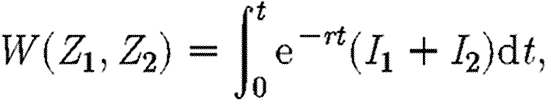 |
A11 |
where the integral is evaluated along the path defined by the ‘treat-all’ propagation equations (2.1) and starting from the point 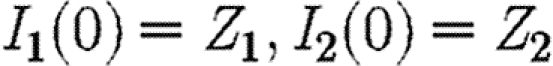 . The transversality conditions for a path that enters this set are as follows:
. The transversality conditions for a path that enters this set are as follows:
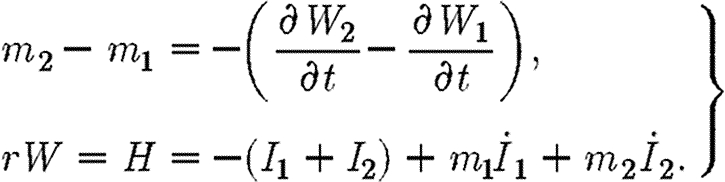 |
A12 |
A.2. The singular solution
Suppose that the control variables are chosen from the interior of their domains so that 0<Fi<Ii. This implies that xi=yi=0 for i=1, 2, and hence from (A 3) it follows that m2=m1. Suppose also that the latter equality holds throughout an open interval of time. Then  from which it is simple to show that I1=I2, whence
from which it is simple to show that I1=I2, whence 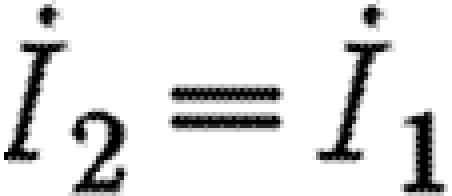 and F2=F1=M/2c. This yields the ‘singular’ solution, which is given by
and F2=F1=M/2c. This yields the ‘singular’ solution, which is given by
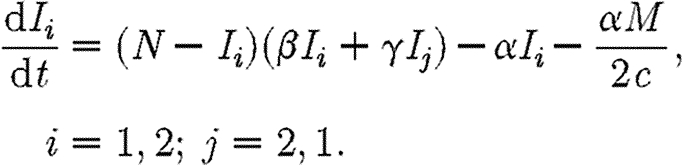 |
A13 |
A.3. The most rapid approach path
The MRAP involves reaching the singular solution as fast as possible and remaining on this solution thereafter. This means giving preferential treatment to the subpopulation with the higher prevalence of disease until disease in the two populations is equalized, and then treating these populations equally. This strategy implies that
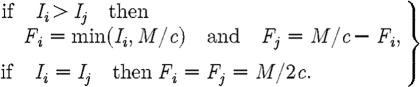 |
A14 |
To confirm that this strategy is the worst case, we show that this path maximizes the discounted level of infection (equation (2.3)). The Hamiltonian is identical to equation (A 4) save that the first term 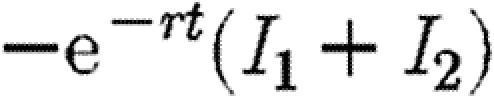 is replaced by
is replaced by 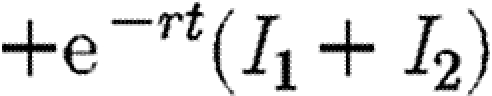 for maximization rather than minimization of the integral V. Hence, the conditions on F1 for maximization are identical to equation (A 5), only this time the co-state variables are positive. Mangasarian's sufficiency conditions for a maximum require that the Hamiltonian be a concave function of I1, I2 and F1 (Seierstad & Sydsaeter 1987). These conditions require that the following matrix is negative semi-definite:
for maximization rather than minimization of the integral V. Hence, the conditions on F1 for maximization are identical to equation (A 5), only this time the co-state variables are positive. Mangasarian's sufficiency conditions for a maximum require that the Hamiltonian be a concave function of I1, I2 and F1 (Seierstad & Sydsaeter 1987). These conditions require that the following matrix is negative semi-definite:
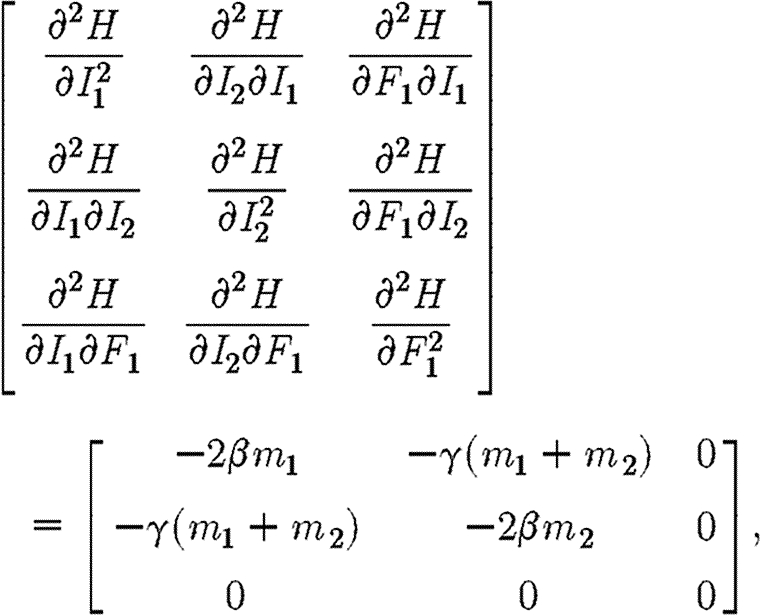 |
A15 |
which will be the case if m1≥0 and
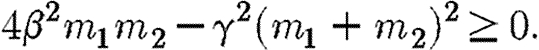 |
A16 |
Provided β>γ, the above inequality is always strictly satisfied on the singular solution, where m1=m2. By continuity, it must also be weakly satisfied on the MRAP for points close to the singular solution. The co-state variables measure the marginal value of additional infection, which in the maximization version of the problem is always positive. Thus m1, m2>0. Moreover, for the parameter values we consider (β>γ), the inequality given in equation (A 16) is also satisfied along the whole length of the trajectory of the singular solution, where m1=m2. These conditions establish that the Hamiltonian is strictly concave on the singular solution and also close to it. For paths that remain permanently outside of the region 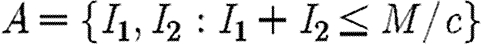 , the transversality conditions
, the transversality conditions 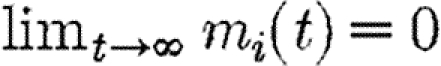 for i=1, 2 are satisfied. For paths that enter this region, the transversality condition m1=m2 at the point where they enter is satisfied. For paths of this type, an additional concavity condition is required (Seierstad & Sydsaeter 1987). Simulations indicate that W(Z1,Z2) is concave. Under these conditions, the MRAP maximizes the integral V and is therefore as bad as, or worse than, any other path that satisfies the constraints of the problem (Seierstad & Sydsaeter 1987).
for i=1, 2 are satisfied. For paths that enter this region, the transversality condition m1=m2 at the point where they enter is satisfied. For paths of this type, an additional concavity condition is required (Seierstad & Sydsaeter 1987). Simulations indicate that W(Z1,Z2) is concave. Under these conditions, the MRAP maximizes the integral V and is therefore as bad as, or worse than, any other path that satisfies the constraints of the problem (Seierstad & Sydsaeter 1987).
A.4. Finding the optimal path
We propose an alternative candidate for the optimal path, when (I1+I2)>M/c. The path is determined by the following decision rules:
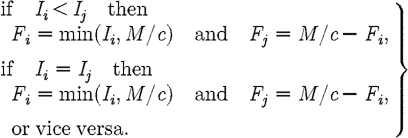 |
A17 |
This path is the anti-MRAP in which preference is given to treating the subpopulation with lower prevalence of infection. Standard sufficiency theorems cannot be used to prove analytically that this is the optimal path since m1<0 and the Hamiltonian is not concave. However, simulations indicate that the anti-MRAP is in fact optimal (see §S2 in the electronic supplementary material).
Using a 100 × 100 grid of starting points we compared the following three paths: path 1, which always gives priority to region 1; path 2, which always gives priority to region 2; and the MRAP, which equalizes infection levels in the two regions as fast as possible and then splits the drug equally between them. Starting from an initial point with I1<I2, the smallest integral was obtained with path 1, the next smallest with path 2 and the strictly largest integral with the MRAP. From an initial point with I2<I1, the smallest integral was obtained with path 2 and the strictly largest integral with the MRAP. With I1=I2 initially, the MRAP always gave the strictly largest integral, but paths 1 and 2 gave identical integrals. We were also able to show that the three paths described above were the only paths that entered the treat-all set (given a suitable starting point) and satisfied both the Hamiltonian and transversality conditions. This suggests that the anti-MRAP strategy of favouring the least infected area is the best. We were not able to rule out the possibility that there are other paths that yield an even lower integral than the anti-MRAP, but we were not able to locate such a path from any starting point.
A.5. Quarantine
The Hamiltonian for the case with quarantine is the same as in equation (A 4) except that γ is replaced by γ0h(Q) as in equation (2.4) and the objective function by equation (2.5). The decision rules are identical to equation (A 17), with the additional constraint that the quarantine variable Q is selected from the set [0, Qmax] so as to maximize the Hamiltonian, taking all other variables as given. The optimal value of Q is thus equal to
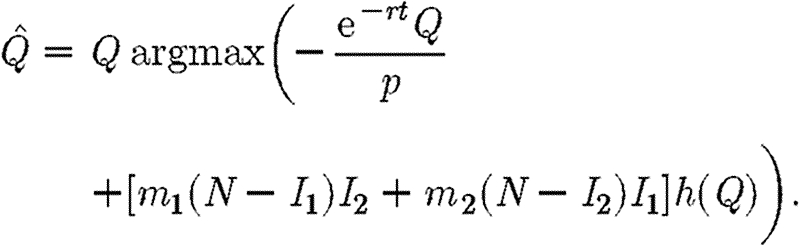 |
A18 |
Note that in this case the discount rate does affect the optimal strategy.
A.6. Error of worst relative to best path
The error ratio for the worst compared with the best paths is computed by (Vworst−Vbest)/Vbest in which Vworst and Vbest are the values of the discounted infection along the best and worst paths, respectively. We distinguish between the maximum and the average value (computed as the average error over all starting points in infection space for which (I1+I2)>M/c for given ratios of transmission between and within subpopulations). The instability region in which the best path leads to disease control and the worst to explosive spread shown in figure 2 were computed for each of 21×21 starting points laid out on a uniform grid on the infection space. When γ=β, the two regions are effectively a single region and all allowable treatment policies lead to exactly the same trajectory for total infection, and hence to the same value for the integral V (equation (2.3)).
References
- Anderson R.M., May R.M. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press [Google Scholar]
- Barrett S. 2003. Global disease eradication. J. Eur. Econ. Assoc. 1, 591–600. 10.1162/154247603322391224 [DOI] [Google Scholar]
- Barrett S., Hoel M. 2007. Optimal disease eradication. Environ. Dev. Econ. 12, 627–652. 10.1017/S1355770X07003816 [DOI] [Google Scholar]
- Dye C., Gay N. 2003. Modeling the SARS epidemic. Science. 300, 1884–1885. 10.1126/science.1086925 [DOI] [PubMed] [Google Scholar]
- Ferguson N.M., Donnelly C.A., Anderson R.M. 2001. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science. 292, 1155–1160. 10.1126/science.1061020 [DOI] [PubMed] [Google Scholar]
- Forster G., Gilligan C.A. 2007. Optimizing the control of disease infestations at the landscape scale. Proc. Natl Acad. Sci. USA. 104, 4984–4989. 10.1073/pnas.0607900104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan C.A. 2003. Economics of transgenic crops and pest resistance, an epidemiological perspective. In Battling resistance to antibiotics and pesticides: an economic approach (ed Laxminarayan R.) pp. 221–243. Washington, DC:Resources for the Future. [Google Scholar]
- Goldman S. M., Lightwood J. 2002. Cost optimisation in the SIS model of infectious disease with treatment. In Topics in economic analysis and policy, vol. 2, pp. 1–24. Berkeley, CA: Berkley Electronic Press. [Google Scholar]
- Grenfell B.T., Bolker B.M. 1998. Cities and villages: infection hierarchies in a measles metapopulation. Ecol. Lett. 1, 63–70. 10.1046/j.1461-0248.1998.00016.x [DOI] [Google Scholar]
- Gyllenberg M., Hanski I., Hastings A. 1997. Structured metapopulation models. In Metapopulation biology: ecology, genetics and evolution (ed Gilpin M.E.) pp. 93–122. San Diego, CA:Academic Press. [Google Scholar]
- Hanski I. 1998. Metapopulation dynamics. Nature. 396, 41–49. 10.1038/23876 [DOI] [Google Scholar]
- Hanski I., Ovaskainen O. 2002. The metapopulation capacity of a fragmented landscape. Nature. 404, 755–758. 10.1038/35008063 [DOI] [PubMed] [Google Scholar]
- Hethcote H.W. 1980. Three basic epidemiological models. In Applied mathematical ecology (ed. Gross L.J.) pp. 19–144. Berlin, Germany:Springer-Verlag. [Google Scholar]
- Keeling M.J., Gilligan C.A. 2000a. Bubonic plague: a metapopulation model of a zoonosis. Proc. R. Soc. B. 267, 2219–2230. 10.1098/rspb.2000.1013 [DOI] [Google Scholar]
- Keeling M.J., Gilligan C.A. 2000b. Metapopulation dynamics of bubonic plague. Nature. 407, 903–906. 10.1038/35038073 [DOI] [PubMed] [Google Scholar]
- Keeling M.J., et al. 2001. Dynamics of the 2001 UK foot and mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science. 294, 813–817. 10.1126/science.1065973 [DOI] [PubMed] [Google Scholar]
- Keeling M.J., Bjørnstad O.N., Grenfell B.T. 2004. Metapopulation dynamics of infectious disease. In Ecology, genetics and evolution of metapopulations (ed. Gaggiotti O.E.) pp. 415–445. Amsterdam, The Netherlands:Elsevier. [Google Scholar]
- Klein E., Laxminarayan R., Smith D.L., Gilligan C.A. 2007. Economic incentives and mathematical models of disease. Environ. Dev. Econ. 12, 707–732. 10.1017/S1355770X0700383X [DOI] [Google Scholar]
- Lajmanovich A., Yorke J.A. 1976. A deterministic model for gonorrhea in a nonhomogeneous population. Math. Biosci. 28, 221–236. 10.1016/0025-5564(76)90125-5 [DOI] [Google Scholar]
- Lipsitch M., Bergstrom C.T., Levin B.R. 2000. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc. Natl Acad. Sci. USA. 97, 1938–1943. 10.1073/pnas.97.4.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.D. 2002. Mathematical biology I: an introduction. Berlin, Germany:Springer-Verlag [Google Scholar]
- Murray G.D., Cliff A.D. 1977. A stochastic model for measles epidemics in a multi-region setting. Trans. Inst. Br. Geogr. 2, 158–174. 10.2307/621855 [DOI] [Google Scholar]
- Murray C.J.L., Lopez A.D. 1996. The global burden of disease. Geneva, Switzerland:World Health Organisation, Harvard School of Public Health, World Bank [Google Scholar]
- Nold A. 1980. Heterogeneity in disease-transmission modeling. Math. Biosci. 52, 227–240. 10.1016/0025-5564(80)90069-3 [DOI] [Google Scholar]
- Park A.W., Gubbins S., Gilligan C.A. 2003. Extinction times for spatially-structured closed epidemics. Ecol. Lett. 5, 747–755. 10.1046/j.1461-0248.2002.00378.x [DOI] [Google Scholar]
- Pinch E. 1993. Optimal control and the calculus of variations.Oxford, UK:Oxford University Press [Google Scholar]
- Pontryagin L.S., Boltyanski B., Gamkrelidze R., Mishchenko E. 1962. The mathematical theory of optimal processes. New York, NY:Interscience [Google Scholar]
- Rowthorn R.E., Brown G.M. 2003. Using antibiotoics when resistance is renewable. In Battling resistance to antibiotics and pesticides: an economic approach (ed. Laxminarayan R.) pp. 42–62. Washington, DC:Resources for the Future. [Google Scholar]
- Seierstad A., Sydsaeter K. 1987. Optimal control theory with economic applications. Amsterdam, The Netherlands:North Holland [Google Scholar]
- Sethi S. 1974. Quantitative guidelines for communicable disease control program: a complete synthesis. Biometrics. 30, 681–691. 10.2307/2529232 [DOI] [PubMed] [Google Scholar]
- Sethi S. 1978. Optimal control of some simple deterministic epidemic models. J. Oper. Res. Soc. 29, 129–136. 10.1057/jors.1978.27 [DOI] [Google Scholar]
- Smith D.L., Levin S.A., Laxminarayan R. 2005. Strategic interactions in multi-institutional epidemics of antibiotic resistance. Proc. Natl Acad. Sci. USA. 102, 3153–3158. 10.1073/pnas.0409523102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A.J., Truscott J.E., Asher M.J.C., Gilligan C.A. 2004. A model for the invasion of rhizomania in the United Kingdom: implications for control strategies. Phytopathology. 94, 209–215. 10.1094/PHYTO.2004.94.2.209 [DOI] [PubMed] [Google Scholar]
- Swinton J., Harwood J., Grenfell B.T., Gilligan C.A. 1998. Persistence thresholds for phocine distemper virus infection in harbour seal Phoca vitulina metapopulations. J. Anim. Ecol. 67, 54–68. 10.1046/j.1365-2656.1998.00176.x [DOI] [Google Scholar]