Abstract
Taxonomic homogenization (TH) is the increasing similarity of the species composition of ecological communities over time. Such homogenization represents a form of biodiversity loss and can result from local species turnover. Evidence for TH is limited, reflecting a lack of suitable historical datasets, and previous analyses have generated contrasting conclusions. We present an analysis of woodland patches across a southern English county (Dorset) in which we quantified 70 years of change in the composition of vascular plant communities. We tested the hypotheses that over this time patches decreased in species richness, homogenized, or shifted towards novel communities. Although mean species richness at the patch scale did not change, we found increased similarity in species composition among woodlands over time. We concluded that the woodlands have undergone TH without experiencing declines in local diversity or shifts towards novel communities. Analysis of species characteristics suggested that these changes were not driven by non-native species invasions or climate change, but instead reflected reorganization of the native plant communities in response to eutrophication and increasingly shaded conditions. These analyses provide, to our knowledge, the first direct evidence of TH in the UK and highlight the potential importance of this phenomenon as a contributor to biodiversity loss.
Keywords: biotic homogenization, biodiversity, conservation, environmental change
1. Introduction
Biodiversity loss is occurring widely at local and regional scales, leading to changes in the composition of biological communities (Poiani et al. 2000). Altered composition can result from local species loss, which reduces the number of species present within a habitat patch (lower α-diversity), or from immigration, which increases species richness. At larger spatial scales, such processes can lead to biotic homogenization (BH). BH refers to increasing similarity among communities over time, reflecting changes in species composition caused by local immigration and extinction (McKinney & Lockwood 1999; Castro & Jaksic 2008b). It is generally caused by an increase in the abundance of cosmopolitan or widespread species, which may be accompanied by a decrease in the abundance of more specialist or rare species (Castro & Jaksic 2008a). BH has attracted increasing research interest in the context of understanding the impacts of environmental change on biodiversity because of its potential role in the loss of specific community types and biotic impoverishment (Olden et al. 2004; Rooney et al. 2007). Despite such interest, understanding of BH remains limited.
The evidence for BH is highly variable and sometimes conflicting. Such variation can be attributed partly to the occurrence of different types of BH: genetic, taxonomic and functional (Olden & Rooney 2006). Genetic homogenization refers to an increased similarity between gene pools as a result of hybridization or genetic bottlenecks. Functional homogenization (FH) refers to an increase in the similarity of species’ functional ‘roles’ across communities and is the type most strongly supported by evidence (Olden & Poff 2004; Olden & Rooney 2006). FH has been observed in plant communities in Britain, being attributed to expansion of historically contingent species with ‘winning’ traits in response to land-use change (Smart et al. 2006).
Taxonomic homogenization (TH) refers to an increase in similarity of species composition across a set of communities (Olden & Rooney 2006). If TH occurs, this will often indicate FH, but also indicates a decrease in β-diversity. Most commonly, TH has been documented as the result of the spread of non-native species across a region, resulting in the loss of native species (McKinney & Lockwood 1999). Evidence for TH comes primarily from regional or national comparisons of the similarity between introduced and native species pools (Castro et al. 2007; Hoagstrom et al. 2007; McKinney & La Sorte 2007; Magee et al. 2008) and community similarities along large-scale spatial gradients (Kuhn & Klotz 2006; Dormann et al. 2007; Blair & Johnson 2008). However, contrasting evidence is also available (Beck & Khen 2007). For example, species invasions were found not to simplify taxonomic composition of Mediterranean floras (Lambdon et al. 2008), and the flora of two US states showed more instances of differentiation than homogenization (Qian et al. 2008).
Conflicting evidence for TH partly reflects variation in the methods used. The most powerful method for detecting TH involves the comparison of complete species pools from the same sites at different times (Olden & Rooney 2006). This method has rarely been employed because it is dependent on the availability of suitable historical data, which are usually lacking (Olden & Rooney 2006). Application of this approach demonstrated TH over five decades in Wisconsin (USA) woodland plant communities as a result of local extinction (Rooney et al. 2004). In contrast, Smart et al. (2006) found no evidence of taxonomic impoverishment in a sample of plant communities throughout Britain over a 20-year period. Different results have therefore been obtained in terms of both the occurrence of TH and the processes responsible. Variation among studies might also be attributable to the contrasting time scales investigated.
In this investigation, we analysed changes in the composition of woodland vascular plant communities using surveys undertaken at an interval of seven decades. This represents the longest time period over which TH has been assessed by examining changes in composition at the same sites. Specifically, we tested the following (not mutually exclusive) hypotheses: (i) habitat patches from the two survey times do not differ significantly in species composition (null hypothesis); (ii) species richness of patches has decreased over time; (iii) patches have become significantly more similar to each other over time (demonstrating TH); (iv) communities present in 2008 are a subset of those present in the 1930s, and therefore novel communities have not developed over this time period. We examined the potential processes responsible for any observed composition changes by testing whether; (v) plant traits indicate that environmental conditions (soil fertility, the degree of shading, soil pH, soil moisture, air temperature) had changed between the two surveys; and (vi) the proportion of non-native species had increased over time.
2. Methodology
(a). The ‘Good’ survey
We used a dataset created in the 1930s, which provides a rare opportunity to examine changes in species composition over seven decades. From 1931 to 1939, Ronald Good undertook a survey of vascular plant species at 7575 sites throughout the southern English county of Dorset. Good selected sites using what he referred to as the ‘stand’ method. Stands were ‘ … reasonably distinct topographical and ecological entit[ies] … ’ and were required to be ‘ … as evenly scattered as possible’ across Dorset (Good 1937). Stands varied in size from 0.5 to 20 ha and were surveyed by recording all vascular plant species encountered during a survey of approximately 1 h. Stand locations were recorded on a series of 6 inch Ordnance Survey (OS) maps, which were subsequently digitized by the Dorset Environmental Records Centre (DERC). Each patch was visited once, generating presence–absence data that are relatively robust to sampling error (Hirst & Jackson 2007).
(b). Resurvey of woodland patches
For clarity, henceforth we refer to Good's stands as ‘patches’ and the species list for a patch as a ‘community’. We resurveyed a selection of the patches classified as woodland by Horsfall (1989). We selected a sample of patches for potential resurvey by determining which were still extant and had not been replanted, using current maps of woodland habitat provided by DERC. Selected woodlands corresponded to ancient seminatural woodland habitats (Natural England 2003) and priority habitats of lowland mixed deciduous woodland, lowland beech and yew, wet woodland and wood pasture and parkland (BRIG 2008). Of the resulting 592 selected patches, a sample of 86 were randomly selected for resurvey. During the survey process, 21 patches were found to be inaccessible and were replaced by new samples.
Patches were relocated in the field using a Global Positioning System (eTrex venture, Garmin Ltd, Southampton, UK) supported by digital maps of the Good patches derived from DERC and 1 : 25 000 scale raster OS tiles (Ordnance Survey 2009). Each patch was surveyed on a day and month as close as possible to the dates employed by Good in the 1930s and was searched over approximately 2 h to minimize errors of species loss. A single person carried out all surveys. All vascular plant species were identified in situ or by removing or photographing specimens for expert determination. A few plants were identified only to genus by Good and were assumed to be the same species as specimens of that genus found in a patch in 2008.
(c). Data analysis
To identify any bias caused by different sampling dates, changes in species number within each patch between the two surveys were correlated against the number of days between the Good survey date and the resurvey date. We performed analysis of similarity (ANOSIM) with 1000 permutations using the ‘vegan’ package (Oksanen et al. 2008) in R v. 2.8.1 (R Development Core Team 2008) to determine whether species composition of patches differed between the two surveys. We then used Sørensen similarity indices (S) to assess the homogenization of, and any shift in, community composition within patches between the surveys (Shaw 2003).
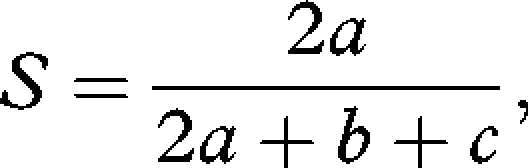 |
2.1 |
where a is the total number of species present in both patches being compared, b is the number of species present only in patch 1 and c is the number of species present only in patch 2.
To determine changes in variation among the communities, we calculated the Sørensen index between all community pairs using the Good data. This resulted in 85 indices for each community, from which we calculated the mean Sørensen index for each community x, which was designated Six. We repeated these steps for the 2008 resurvey data, which provided measures of the variation among the communities in 2008, Sjx. To determine the extent of the changes between the Good survey and the resurvey, we calculated the mean of the indices for each 2008 community compared with all 1930s communities, Skx. In the event of homogenization, we would expect Sjx > Six. In the event of differentiation, we would expect Six > Sjx. In the event of a shift in community composition, Skx would have the lowest value. The non-parametric Kendall's W-test for multiple related dependent samples was used to compare Six, Sjx and Skx.
We also assessed community changes by multivariate detrended correspondence analysis (DCA) using the vegan package (Oksanen et al. 2008) in R v. 2.8.1 (R Development Core Team 2008). The number of axis rescalings was set at the default of four and segments as 26 (Oksanen 2008). Outliers were detected by the Mahalanobis distance of the four DCA axes on the first run (Tabachnick & Fidell 2001). Communities that exceeded the critical Mahalanobis value of 18.47 (for p < 0.001, d.f. = 4) were removed, along with their corresponding patch pair. As a result, the number of samples included in the analysis was reduced by five patches and the number of species was reduced by 16. The ‘ellipse’ package (Murdoch et al. 2007) was used to create bivariate standard deviational ellipses for DCA axes 1 and 2 at 90 per cent confidence intervals. Ellipses were created for the Good data and for the resurvey data.
We examined the potential processes behind observed composition changes by using scores of plant traits that indicate tolerance of different environmental conditions. Trait scores for all species were obtained from PLANTATT (Hill et al. 2004) and were used to create a mean value for each trait for each patch in each survey. The traits examined provide an indication of soil fertility (Ellenberg N), the degree of shading (Ellenberg L), soil pH (Ellenberg R), soil moisture (Ellenberg F) and climate (mean January and mean July temperatures; see Hill et al. (2004) for calculation details). In addition, species were classified as either native or non-native (archaeophytes, neophytes and casuals) to determine any changes in the proportion of non-native species between the surveys.
3. Results
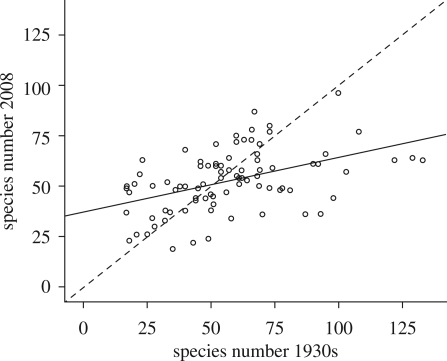
There was no correlation between the changes in species number within each patch and the number of days between the dates of the two surveys (rs = 0.11; p = 0.33), suggesting that any differences in survey date did not bias the results. The ANOSIM suggested a highly significant difference between habitat patches from the two survey times (R = 0.026, p = 0.001). Comparison of species lists across all sites showed that 117 species were lost and 47 species were gained between the 1930s and 2008 surveys. However, the mean (± s.e.) number of species per patch did not change significantly between the 1930s (57 ± 2.8) and 2008 (53 ± 1.6) (χ2 = 0.145, d.f. = 1, p = 0.70), indicating no significant biotic impoverishment at the patch level. Patches with a relatively high species number in the 1930s tended to show decreased species number by 2008, and the converse was also true (figure 1). As a result, the species number of patches converged between the surveys. This was demonstrated by linear regression, which indicated a significant influence of species number per patch obtained in the 1930s on that in 2008, with the gradient of the regression line being less than 1 (figure 1).
Figure 1.
Species numbers at each survey time. The dashed line represents the null hypothesis of no change in species number of a community between the two surveys. The solid line represents the fitted relationship (r2 =0.209; d.f.=85; p < 0.001), which suggests that sites with communities with high species number in the 1930s have tended to decrease over time and those with a low species number in the 1930s have increased over time. The gradient of the regression was significantly different to 1 (gradient 0.27; 95% confidence intervals 0.16, 0.39).
The Sørensen indices for the 1930s (mean across all x patches, 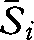 = 32.8), for 2008 (
= 32.8), for 2008 (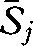 = 46.6) and for the 1930s versus 2008 (
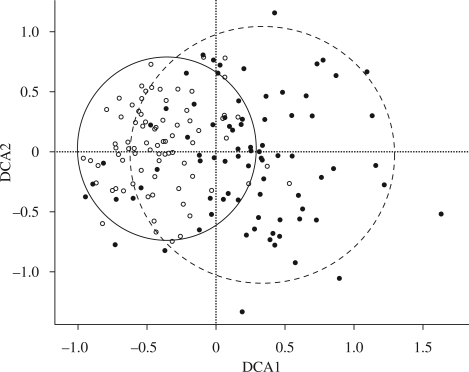
= 46.6) and for the 1930s versus 2008 (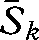 = 35.1) were significantly different from each other (W = 0.728, d.f. = 2, p < 0.001). This suggests greater taxonomic homogeneity in 2008 than in the 1930s, a finding supported by the DCA, which indicated both a reduction in variation and a shift in composition between the surveys (figure 2). Eigenvalues for the DCA were 0.25 and 0.17 for axes 1 and 2, respectively. Wilcoxon tests showed that the difference between the median community scores for the 1930s and 2008 surveys were significant for axis 1 (T = 159, p < 0.001) and axis 2 (T = 542, p < 0.001). The 90 per cent confidence interval ellipses for axes 1 and 2 showed strong overlap of the 1930s and 2008 communities, indicating that novel communities have not developed over this time period (figure 2).
= 35.1) were significantly different from each other (W = 0.728, d.f. = 2, p < 0.001). This suggests greater taxonomic homogeneity in 2008 than in the 1930s, a finding supported by the DCA, which indicated both a reduction in variation and a shift in composition between the surveys (figure 2). Eigenvalues for the DCA were 0.25 and 0.17 for axes 1 and 2, respectively. Wilcoxon tests showed that the difference between the median community scores for the 1930s and 2008 surveys were significant for axis 1 (T = 159, p < 0.001) and axis 2 (T = 542, p < 0.001). The 90 per cent confidence interval ellipses for axes 1 and 2 showed strong overlap of the 1930s and 2008 communities, indicating that novel communities have not developed over this time period (figure 2).
Figure 2.
DCA bi-plot for communities. Filled circles are 1930s communities, open circles are 2008 communities. The plot depicts bivariate standard deviational ellipses for axes 1 and 2 at a confidence interval of 90% for each survey time. The ellipse to the right is for 1930s communities and to the left is for 2008 communities. The ellipses overlap and show a shift of communities to the left of the plot over time.
Changes in the mean plant trait values per patch between the two surveys suggested highly significant (p < 0.001, Wilcoxon matched pairs tests) increases in soil fertility and in shading (table 1), but there were no significant differences in traits relating to air temperature, soil pH or soil moisture. The proportion of non-native species within a patch increased significantly over time (p = 0.019, Wilcoxon matched pairs test), but the proportion was extremely low in both surveys (≤0.03).
Table 1.
Wilcoxon's matched pairs test on mean trait scores for patches. Overall mean patch scores for each survey are provided to indicate the direction of change in traits.
| trait | mean 1930s patch score | mean 2008 patch score | T | p |
|---|---|---|---|---|
| Ellenberg L (light) | 5.80 | 5.44 | 465 | <0.001 |
| Ellenberg N (fertility) | 5.02 | 5.44 | 229 | <0.001 |
| Ellenberg R (pH) | 5.87 | 5.93 | 1434 | 0.060 |
| Ellenberg F (moisture) | 5.57 | 5.55 | 1814 | 0.808 |
| mean January temperature (°C) | 3.52 | 3.52 | 1787 | 0.719 |
| mean July temperature (°C) | 14.51 | 14.52 | 1779 | 0.694 |
| proportion of non-native species | 0.02 | 0.03 | 981 | 0.019 |
4. Discussion
This study provides clear evidence of changed composition of the woodland flora over a 70-year period. The effect of this change has been TH, as indicated by the increase in Sørensen indices over time and the reduction in variation detected by DCA. We therefore accept the hypothesis that patches have increased in similarity in terms of their species composition, demonstrating TH. To our knowledge, this is the first time that TH has been documented in the UK. Our result contrasts with the analysis of Smart et al. (2006), which found no evidence for TH among a large number of habitats throughout Britain over 20 years, but did find evidence for FH, which was attributed to the expansion of species with similar winning traits. The contrasting results obtained here may reflect the different time spans over which the comparison was made, with the current study being longer than that of any previous study using the same method.
Contrary to previous studies where TH has been identified (Rooney et al. 2004), the observed TH was not associated with a decline in the mean number of species per habitat patch. However, there was a decline in species number at the landscape scale between the surveys. While some previous studies of changes in British plant communities detected losses of α-diversity (e.g. Stevens et al. 2004), others did not. Smart et al. (2006) observed increased floristic species richness in some habitat patches over two decades in Britain, which was attributed to initial habitat productivity and disturbance. Within the Dorset woodland patches, the spread of species with traits more suited to the changed environment appears to have been matched by declines in other species, leading to a balance between the number of colonizations and extinctions.
The large overlap of DCA ellipses and the relatively high similarity of communities between the two surveys (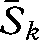 ) support the hypothesis that the woodland communities have not shifted towards novel compositions, an expected response to climate change (Williams & Jackson 2007; Keith et al. in press), and that those communities found in 2008 were a subset of those occurring in the 1930s. This result suggests that the Dorset woodlands are not yet showing any response to recent climate change, a conclusion supported by the lack of climate-related changes in the plant trait analyses.
) support the hypothesis that the woodland communities have not shifted towards novel compositions, an expected response to climate change (Williams & Jackson 2007; Keith et al. in press), and that those communities found in 2008 were a subset of those occurring in the 1930s. This result suggests that the Dorset woodlands are not yet showing any response to recent climate change, a conclusion supported by the lack of climate-related changes in the plant trait analyses.
Analysis of plant traits suggested that the TH observed reflected reorganization of the native plant communities in response to eutrophication (Ellenberg N) and increasingly shaded conditions (Ellenberg L). Eutrophication has previously been associated with local extinctions (Walker & Preston 2006) and changes in communities of native plant species (Portejoie et al. 2002) in the UK, and Smart et al. (2006) implicated eutrophication and changes in anthropogenic disturbance in the FH of British plant communities. The increasingly shaded conditions of woodlands in the UK can be attributed to the widespread decline in traditional woodland management since the 1930s (Kirby et al. 2005). Van Calster et al. (2007) showed similar management-driven TH within a Belgian forest in a comparison of coppice-with-standards forest with high forest.
The results of the current investigation therefore indicate that TH can be caused by environmental change without contribution from non-native species. While the proportion of non-native species increased significantly between the surveys, it was extremely low in both. Much previous research into different types of BH has focused on invasion by non-native species as a principal cause (Castro et al. 2007; Hoagstrom et al. 2007; McKinney & La Sorte 2007; Magee et al. 2008). While non-native species can cause large losses of native species in some circumstances (Maskell et al. 2006a), national surveys accord with the results of this study in showing that non-natives are minor components of many British plant communities (Maskell et al. 2006b).
In conclusion, Dorset woodlands have undergone TH over the last 70 years, with no floristic impoverishment at the patch scale, nor any shift towards new community compositions. Changes are attributable to a reorganization of native plant communities, possibly in response to increasing eutrophication and more shaded conditions. The contrast between scales of measurement illustrates the value of landscape-scale (β-diversity) analyses in detecting biodiversity loss that might otherwise go unnoticed. Our findings also highlight the importance of changes in native species distributions, which is a more subtle floristic change than invasion of non-natives, but is of equal consequence to biodiversity loss at higher levels of the organization (Cassey et al. 2008).
Acknowledgements
We would like to thank the Dorset Environmental Records Centre (DERC) for supplying the Professor Good Archive records and stand location data. We would also like to thank R. Clarke and D. Golicher for providing very helpful assistance with statistical analysis, and M. Hill for helpful discussion of the data. We are extremely grateful to L. Davy for assistance with plant identification.
References
- Beck J., Khen C. V.2007Beta-diversity of geometrid moths from northern Borneo: effects of habitat, time and space. J. Anim. Ecol. 76, 230–237 (doi:10.1111/j.1365-2656.2006.01189.x) [DOI] [PubMed] [Google Scholar]
- Blair R. B., Johnson E. M.2008Suburban habitats and their role for birds in the urban–rural habitat network: points of local invasion and extinction? Landsc. Ecol. 23, 1157–1169 (doi:10.1007/s10980-008-9267-y) [Google Scholar]
- BRIG. UK biodiversity action plan: priority habitat descriptions. 2008. See http://www.ukbap.org.uk/PriorityHabitats.aspx .
- Cassey P., Lockwood J. L., Olden J. D., Blackburn T. M.2008The varying role of population abundance in structuring indices of biotic homogenization. J. Biogeogr. 35, 884–892 (doi:10.1111/j.1365-2699.2007.01827.x) [Google Scholar]
- Castro S. A., Jaksic F. M.2008aHow general are global trends in biotic homogenization? Floristic tracking in Chile, South America. Glob. Ecol. Biogeogr. 17, 524–531 (doi:10.1111/j.1466-8238.2008.00392.x) [Google Scholar]
- Castro S. A., Jaksic F. M.2008bRole of non-established plants in determining biotic homogenization patterns in Pacific Oceanic Islands. Biol. Invasions 10, 1299–1309 (doi:10.1007/s10530-007-9204-z) [Google Scholar]
- Castro S. A., Munoz M., Jaksic F. M.2007Transit towards floristic homogenization on oceanic islands in the south-eastern Pacific: comparing pre-European and current floras. J. Biogeogr. 34, 213–222 (doi:10.1111/j.1365-2699.2006.01605.x) [Google Scholar]
- Dormann C. F., et al. 2007Effects of landscape structure and land-use intensity on similarity of plant and animal communities. Glob. Ecol. Biogeogr. 16, 774–787 (doi:10.1111/j.1466-8238.2007.00344.x) [Google Scholar]
- Good R.1937An account of a botanical survey of Dorset. Proc. Linn. Soc. 149, 114–116 [Google Scholar]
- Hill M. O., Preston C. D., Roy D. B.2004PLANTATT. Attributes of British and Irish plants: status, size, life history, geography and habitats for use in connection with the new atlas of the British and Irish flora Wallingford, UK: Centre for Ecology and Hydrology [Google Scholar]
- Hirst C. N., Jackson D. A.2007Reconstructing community relationships: the impact of sampling error, ordination approach, and gradient length. Divers. Distrib. 13, 361–371 (doi:10.1111/j.1472-4642.2007.00307.x) [Google Scholar]
- Hoagstrom C. W., Wall S. S., Kral J. G., Blackwell B. G., Berry C. R.2007Zoogeographic patterns and faunal change of south Dakota fishes. West. North Am. Nat. 67, 161–184 (doi:10.3398/1527-0904(2007)67[161:ZPAFCO]2.0.CO;2) [Google Scholar]
- Horsfall A.1989Some effects of habitat change on the Dorset flora. Proc. Dorset Nat. Hist. Arch. Soc. 111, 99–104 [Google Scholar]
- Keith S. A., Newton A. C., Morecroft M. D., Herbert R. J. H., Bealey C. E.In press Non-analogous community formation in response to climate change. J. Nat. Conserv (doi:10.1016/j.jnc.2009.04.003) [Google Scholar]
- Kirby K. J., Smart S. M., Black H. I. J., Bunce R. G. H., Corney P. M., Smithers R. J.2005Long term ecological change in British woodland (1971–2001). A re-survey and analysis of change based on the 103 sites in the Nature Conservancy ‘Bunce 1971’ woodland survey. In English Nature research reports, p. 137 Peterborough, UK: English Nature [Google Scholar]
- Kuhn I., Klotz S.2006Urbanization and homogenization: comparing the floras of urban and rural areas in Germany. Biol. Conserv. 127, 292–300 (doi:10.1016/j.biocon.2005.06.033) [Google Scholar]
- Lambdon P. W., Lloret F., Hulme P. E.2008Do non-native species invasions lead to biotic homogenization at small scales? The similarity and functional diversity of habitats compared for alien and native components of Mediterranean floras. Divers. Distrib. 14, 774–785 (doi:10.1111/j.1472-4642.2008.00490.x) [Google Scholar]
- Magee T. K., Ringold P. L., Bollman M. A.2008Alien species importance in native vegetation along wadeable streams, John Day River basin, Oregon, USA. Plant Ecol. 195, 287–307 (doi:10.1007/s11258-007-9330-9) [Google Scholar]
- Maskell L. C., Bullock J. M., Smart S. M., Thompson K., Hulme P. E.2006aThe distribution and habitat associations of non-native plant species in urban riparian habitats. J. Veg. Sci. 17, 499–508 [Google Scholar]
- Maskell L. C., Firbank L. G., Thompson K., Bullock J. M., Smart S. M.2006bInteractions between non-native plant species and the floristic composition of common habitats. J. Ecol. 94, 1052–1060 (doi:10.1111/j.1365-2745.2006.01172.x) [Google Scholar]
- McKinney M. L., La Sorte F. A.2007Invasiveness and homogenization: synergism of wide dispersal and high local abundance. Glob. Ecol. Biogeogr. 16, 394–400 (doi:10.1111/j.1466-8238.2007.00296.x) [Google Scholar]
- McKinney M. L., Lockwood J. L.1999Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (doi:10.1016/S0169-5347(99)01679-1) [DOI] [PubMed] [Google Scholar]
- Murdoch D., Chow E. D. & Porting to R by Frias Celayeta, J. M. 2007Ellipse: functions for drawing ellipses and ellipse-like confidence regions R package version 0.3–5. See http://cran2.arsmachinandi.it/src/contrib/descriptions/ellipse.html
- Natural England. Ancient woodland inventory (provisional) for England—digital boundaries. 2003. See http://www.english-nature.org.uk/pubs/gis/tech_aw.htm .
- Oksanen J. Multivariate analysis of ecological communities in R: vegan tutorial. 2008. See http://cc.oulu.fi/~jarioksa/opetus/metodi/vegantutor.pdf .
- Oksanen J., Kindt R., Legendre P., O'Hara B., Simpson G. L., Solymos P., Henry M., Stevens H., Wagner H.2008Vegan: community ecology package, R package version 1.15–1. See http://vegan.r-forge.r-project.org/: http://cran.r-project.org/
- Olden J. D., Poff N. L.2004Ecological processes driving biotic homogenization: testing a mechanistic model using fish faunas. Ecology 85, 1867–1875 (doi:10.1890/03-3131) [Google Scholar]
- Olden J. D., Rooney T. P.2006On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 15, 113–120 (doi:10.1111/j.1466-822X.2006.00214.x) [Google Scholar]
- Olden J. D., Poff N. L., Douglas M. R., Douglas M. E., Fausch K. D.2004Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19, 18–24 (doi:10.1016/j.tree.2003.09.010) [DOI] [PubMed] [Google Scholar]
- Ordnance Survey 20091 : 25 000 scale colour raster Southampton, UK: Ordnance Survey; See http://edina.ac.uk/digimap/ [Google Scholar]
- Poiani K. A., Richter B. D., Anderson M. G., Richter H. E.2000Biodiversity conservation at multiple scales: functional sites, landscapes, and networks. Bioscience 50, 133–146 (doi:10.1641/0006-3568(2000)050[0133:BCAMSF]2.3.CO;2) [Google Scholar]
- Portejoie S., Martinez J., Landmann G.2002Ammonia of farm origin: impact on human and animal health and on the natural habitat. Prod. Anim. 15, 151–160 [Google Scholar]
- Qian H., McKinney M. L., Kuhn I.2008Effects of introduced species on floristic similarity: comparing two US states. Basic Appl. Ecol. 9, 617–625 (doi:10.1016/j.baae.2007.11.004) [Google Scholar]
- R Development Core Team 2008R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org [Google Scholar]
- Rooney T. P., Wiegmann S. M., Rogers D. A., Waller D. M.2004Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv. Biol. 18, 787–798 (doi:10.1111/j.1523-1739.2004.00515.x) [Google Scholar]
- Rooney T. P., Olden J. D., Leach M. K., Rogers D. A.2007Biotic homogenization and conservation prioritization. Biol. Conserv. 134, 447–450 (doi:10.1016/j.biocon.2006.07.008) [Google Scholar]
- Shaw P.2003Multivariate statistics for the environmental sciences New York, NY: Hodder Arnold [Google Scholar]
- Smart S. M., Thompson K., Marrs R. H., Le Duc M. G., Maskell L. C., Firbank L. G.2006Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B 273, 2659–2665 (doi:10.1098/rspb.2006.3630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. J., Dise N. B., Mountford J. O., Gowing D. J.2004Impact of nitrogen deposition on the species richness of grasslands. Science 303, 1876–1879 (doi:10.1126/science.1094678) [DOI] [PubMed] [Google Scholar]
- Tabachnick B. G., Fidell L. S.2001Cleaning up your act: screening data prior to analysis. In Using multivariate statistics, 4th edn (eds Tabachnick B. G., Fidell L. S.), pp. 56–110 Boston, MA: Allyn and Bacon [Google Scholar]
- Van Calster H., Baeten L., De Schrijver A., De Keersmaeker L., Rogister J. E., Verheyen K., Hermy M.2007Management driven changes (1967–2005) in soil acidity and the understorey plant community following conversion of a coppice-with-standards forest. Forest. Ecol. Manag. 241, 258–271 (doi:10.1016/j.foreco.2007.01.007) [Google Scholar]
- Walker K. J., Preston C. D.2006Ecological predictors of extinction risk in the flora of Lowland England, UK. Biodivers. Conserv. 15, 1913–1942 (doi:10.1007/s10531-005-4313-4) [Google Scholar]
- Williams J. W., Jackson S. T.2007Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482 (doi:10.1890/070037) [Google Scholar]