Abstract
The structure of plant–pollinator networks has been claimed to be resilient to changes in species composition due to the weak degree of dependence among mutualistic partners. However, detailed empirical investigations of the consequences of introducing an alien plant species into mutualistic networks are lacking. We present the first cross-European analysis by using a standardized protocol to assess the degree to which a particular alien plant species (i.e. Carpobrotus affine acinaciformis, Impatiens glandulifera, Opuntia stricta, Rhododendron ponticum and Solanum elaeagnifolium) becomes integrated into existing native plant–pollinator networks, and how this translates to changes in network structure.
Alien species were visited by almost half of the pollinator species present, accounting on average for 42 per cent of the visits and 24 per cent of the network interactions. Furthermore, in general, pollinators depended upon alien plants more than on native plants. However, despite the fact that invaded communities received more visits than uninvaded communities, the dominant role of alien species over natives did not translate into overall changes in network connectance, plant linkage level and nestedness. Our results imply that although supergeneralist alien plants can play a central role in the networks, the structure of the networks appears to be very permeable and robust to the introduction of invasive alien species into the network.
Keywords: alien plants, invader impact, mutualistic relationships, nestedness, supergeneralist plants
1. Introduction
Insect-dependent pollination is essential for seed production for many plants, and flowers offer the basic resources (i.e. nectar and pollen) needed for the development of many insects. Plant–pollinator interactions have attracted a lot of attention from ecologists but it has not been until recently that they have been perceived as networks with robust architectural properties (Bascompte et al. 2003, 2006). Plant–pollinator interactions are weak and usually asymmetric, that is, plant species depend strongly on pollinator species that depend weakly on reciprocal plants and vice versa (Bascompte et al. 2006; Petanidou & Potts 2006; Petanidou et al. 2008). Plant–pollinator networks are highly nested consisting of a core of generalist species interacting with both generalist and specialist species; this structure is very cohesive (Bascompte et al. 2003; Ollerton et al. 2003; Blüthgen et al. 2007). This nested structure has been claimed to be very stable to environmental and biotic stochasticity (Petanidou et al. 2008) with low sensitivity to sampling effort (Nielsen & Bascompte 2007). However, the empirical evidence for plant–pollinator network stability in the face of global change is scarce, while most of it is based on models that simulate species removals (Memmott et al. 2004; Rezende et al. 2007) and habitat destruction (Fortuna & Bascompte 2006) as two main drivers of biodiversity loss. Even less explored are the effects of species introductions on network stability (but see Olesen et al. 2002; Aizen et al. 2008; Bartomeus et al. 2008).
Biological invasions can serve as natural experiments in community assembly (Sargent & Ackerly 2008). By comparing naturally invaded and uninvaded communities or by exploring gradients of invasion we can test whether the insertion of a new species to a community can have disrupting effects on the structure of the plant–pollinator network. The establishment of many generalist entomophilous plant invaders suggests that there is an efficient use of native pollinators (Richardson et al. 2000). Furthermore, the analysis of pairwise plant–pollinator interactions demonstrates a predominant detrimental impact of alien plants on pollination of native plants (Morales & Traveset 2009). However, the outcome of pairwise plant–pollinator interactions varies, being most detrimental when alien and focal native species have similar flower symmetry or colour. Nonetheless, despite alien plant species being well integrated into recipient plant–pollinator networks (Memmott & Waser 2002) and the evidence of negative effects on particular native species, it is difficult to predict how changes in pairwise plant–pollinator interactions scale up to modifications of the whole plant–pollinator network.
Empirical explorations of how plant-pollination networks respond to species introductions have been scarce. These studies have compared invaded networks with networks where the invader was experimentally removed and therefore the invader could have a legacy effect (Lopezaraiza-Mikel et al. 2007), or along a gradient of invasion (Morales & Aizen 2002; Olesen et al. 2002; Aizen et al. 2008) where it is difficult to disentangle whether the main drivers of network changes are alien plants or alien pollinators and to determine which particular species are causing most of the effects. In fact, most of the evidence for the presence of alien species having disruptive effects on plant-pollination interactions (Traveset & Richardson 2006) is based on invader complexes in which supergeneralist and abundant invasive pollinators displace native pollinators and are less efficient in pollinating some native plant species. In these previous experimental field studies, comparable historically uninvaded (control) networks are often lacking.
We present the first large-scale study comparing invaded and uninvaded plant–pollinator networks of five invasive plant species representative of different European biomes, viz. Mediterranean, temperate and Atlantic. Our aims are to (i) describe how alien plant invaders integrate into the plant–pollinator network compared with sympatric native plant species which have long coexisted in the community, (ii) determine how this translates to changes in plant–pollinator network structure and (iii) explore whether alien plant integration has negative impacts on native plant pollination. We expect invasive entomophilous plants to be supergeneralists (Richardson et al. 2000), to play a central role in the invaded plant–pollinator network by attracting more pollinators than native plant species (Lopezaraiza-Mikel et al. 2007) and to decrease visitation rates to native plants. Furthermore, we hypothesize that invasive plants by being supergeneralists, both interacting with generalists and specialists, will increase the nestedness of the plant–pollinator network (Aizen et al. 2008).
2. Material and methods
(a). Invasive target species and sites
We explored qualitative plant–pollinator networks from communities uninvaded and invaded by the plant species Rhododendron ponticum L. (Ericaceae) (native to Southern and Eastern Europe) in Ireland, Himalayan Impatiens glandulifera Royle (Balsaminaceae) in Germany, American species Solanum elaeagnifolium Cav. (Solanaceae) in Lesvos Island (Greece) and Opuntia stricta (Haw.) Haw. (Cactaceae) in Spain, and the South-African Carpobrotus affine acinaciformis (Aizoaceae) also in Spain. For simplicity, we refer to these species by their generic names hereafter. All species have been introduced as ornamentals, except for Solanum which was unintentionally introduced with agricultural material. All but one invasive species have a generalized pollination system (Valentine 1978 for Impatiens, Stout et al. 2006 for Rhododendron, Bartomeus & Vilà 2009 for Opuntia and Carpobrotus). Solanum is buzz-pollinated by different groups of bees (Buchmann & Cane 1989).
For each invader species, we sampled three paired sites: three invaded and three uninvaded plots where the invader was absent. No other alien plant species were present in our sites. Each pair had the same vegetation type, similar plant native species richness and composition (table 1). On average, paired invaded and uninvaded plots did not differ in native species richness (sign-rank test, p = 0.27) and species composition was similar (Sørensen index=72.64 ± 4.40%). Paired sites were separated by 500 m to 10 km (table 1). Although the invader species was generally the most dominant species in invaded plots, they had on average a plant cover of less than 40 per cent of the total study area. We chose areas that were not highly invaded to avoid comparing communities in which native plant species have already been displaced.
Table 1.
Main characteristics of invaded and uninvaded plots for five alien plant–pollinator networks. Mean (± s.e.) or minimum and maximum values for three paired communities are indicated.
| plant richness range |
pollinator richness range |
||||||
|---|---|---|---|---|---|---|---|
| alien species | cover (%) | sampling time (min species−1 per plot) | distance between plots (km) | invaded | uninvaded | invaded | uninvaded |
| Carpobrotus | 37.00 ± 6.14 | 36 | 0.5–3 | 10–14 | 9–11 | 22–34 | 18–22 |
| Impatiens | 24.54 ± 5.18 | 62a | 0.1–0.5 | 6–8 | 5–9 | 3–10 | 3–8 |
| Opuntia | 17.33 ± 3.65 | 30 | 0.5–3 | 9–10 | 9–12 | 22–26 | 19–25 |
| Rhododendron | 13.00 ± 2.89 | 120b | 0.9–10 | 7–16 | 11–15 | 24–30 | 27–40 |
| Solanum | 22.08 ± 2.83 | 72 | 0.5–6 | 4–6 | 2–6 | 6–9 | 8–9 |
aThe time is the average per species as the plot was surveyed by a transect walk.
bSome species were subsampled due to rainy weather conditions.
(b). Pollinator sampling
Within each plot, we recorded insects visiting the sexual parts of flowers (hereafter described as ‘pollinators’) to the invader and to all co-flowering plant species present within a representative 50 × 50 m area using a standardized protocol. Plant species from invaded and uninvaded plots were randomly sampled with the same effort (table 1). At each plot, three to six pollinator surveys were carried out at regular intervals during two to six months covering the entire flowering period of the invader. In order to avoid oversampling of the most abundant plant species, all plant species from each study case were sampled for the same amount of time, except for Rhododendron where rain prevented us from sampling all species to the same extent. For this species, visitation data were standardized to the same sampling time per species per plot before analysis.
For Impatiens, we sampled three invaded and three uninvaded 300 m long transects along a riparian habitat within a 30 km2 area. Each transect was visited three times for 135 min per transect from July to August. Therefore, for this species, the sampling effort was different for each flowering plant species (Westphal et al. 2008).
The limited sample size is justified by the considerable effort involved in the simultaneous characterization of several whole networks in areas where reference pollinator collections did not exist. Nevertheless, although increasing the sampling time increases the number of visits observed, it has been shown that the architecture of the plant–pollinator networks is quite robust to sampling effort both in time and space (Nielsen & Bascompte 2007; Petanidou et al. 2008).
Fieldwork was conducted on sunny days with less than 3 m s−1 wind. We only recorded an insect as a visitor if it touched the reproductive organs of the flower. Insects that were not identifiable when visiting the flower were captured and later identified by using existing reference collections and by taxonomic experts. All pollinators were native. Specific sampling details can be found in Bartomeus et al. (2008). Type specimens from each study case are deposited at the Institutes where the co-authors belong.
(c). Plant–pollinator network analysis
We constructed a data matrix for each plot with the total number of visits observed for each plant–pollinator interaction. For each matrix, we calculated plant (P) and pollinator (A) species richness, the relative frequency of visits (V, i.e. visitation rate) and number of interactions (I) to native plants and to the invader. For each matrix, we also calculated network size (M=A × P), connectance (C = 100 * (I/M)) and plant linkage level (I/P).
We also tested for differences in strength between invasive and native plants in the invaded network. The strength of each plant species is a measure of the dependence of pollinators on each particular plant species (Bascompte et al. 2006). Strength is defined as the sum of pollinator dependencies on a particular plant species. Dependence of a pollinator on a plant species (DA,P) is the fraction of all visits by a particular pollinator (A) species to a particular plant (P) and is calculated as DA,P = VA−P/VA, where VA−P is the number of visits of pollinator A to plant P and Va is the total number of visits of pollinator A to all plant species in the community (Bascompte et al. 2006).
To test for the influence of the invader on the structure of the plant–pollinator network, apart from comparing visitation rates, connectance and linkage level between invaded and uninvaded plots, the nestedness index (N) was calculated for each matrix (Atmar & Patterson 1995) as an estimation of plant-pollination network organization (Bascompte et al. 2003). To calculate N, a matrix with pollinators in rows, plants in columns and the presence or absence of interactions in cells was constructed, and the isocline of perfect nestedness was calculated for each matrix. The absence of a pairwise interaction below the isocline and the presence of a pairwise interaction above the isocline were recorded as unexpected. Nestedness is calculated as N = (100−T)/100, where temperature (T) is the normalized measure of global distance from unexpected records to the isocline. N values range from 0 to 1 (maximum matrix nestedness).
To assess the significance of N for each matrix, we compared the observed value of N with a benchmark provided by null model 2 in Bascompte et al. (2003) in which each cell in the interaction matrix has a probability of being occupied that depends equally on the number of interactions of its respective column and row as (Pri + Pci)/2, where Pri is the fraction of interactions of row i and Pci is the fraction of interactions of column i. We generated 1000 random matrices with this null model. Analyses were conducted with the Aninhado software (Guimaraes & Guimaraes 2006). To make N values comparable across different matrix sizes with different connectance we calculated the relative N following Bascompte et al. (2003). Nestedness was only computed for Carpobrotus, Opuntia and Rhododendron as the network sizes (15–80 species) for the other two species was too small to detect a nested plant–pollinator network structure (Bascompte et al. 2003).
We also calculated the contribution of each plant species to idiosyncratic temperature (IT) and compared whether the nestedness contribution (NC = (100−IT)/100) of the invader differed from that of the average value for natives in the invaded network (Selva & Fortuna 2007).
(d). Statistical analysis
We ranked the invader compared with native species in the network for each response parameter (e.g. number of visits, number of interactions, strength, nestedness contribution, etc).
Differences between paired invaded and uninvaded network parameter values (e.g. total number of visits, plant linkage level, connectance, nestedness) were compared with sign-rank tests. Except otherwise noted, median values are given throughout the text. Comparisons with the invaded network were conducted both including and excluding the invader species.
For native species present in paired invaded and uninvaded plots, we compared whether the presence of the invader influenced the number of visits and interactions as response variables by linear mixed effects models fitted by maximum likelihood using the ‘nlme function’ in R, v. 2.8 (R Development Core Team 2008). All response variables fulfilled the requirements of normality and homoscedasticity. Only models based on predictor variables that were not significantly correlated were constructed. Within the mixed models, the factors invader identity, paired-site identity and native plant species were specified as random effects while the presence of the invader was considered as a fixed effect. A nested design was applied with native plant species being nested in paired site and this being nested in invader. The analysis was based on 196 records. The final model was: response variable = presence of the invader (yes or no) + invader identity + native species nested within site + site nested within invader + error.
In addition, we tested the influence of similarity in floral traits on the effect of the invaders on visitation rates and interactions of paired native species. For this purpose, we assigned each native species as either similar or dissimilar to the invader in terms of flower colour (Carpobrotus, Impatiens and Rhododendron: pink; Opuntia: yellow; Solanum: purple) and flower symmetry (Rhododendron and Impatiens: zygomorphic; Carpobrotus, Opuntia and Solanum: actinomorphic). The influence of flower colour and symmetry as two fixed binary factors on the paired differences in the number of visits and interactions to native species were also tested by linear mixed effects models: response variable = flower colour similarity (yes or no) + symmetry similarity (yes or no) + invader identity + native species nested within site + site nested within invader + error.
3. Results
(a). Plant invader versus native species
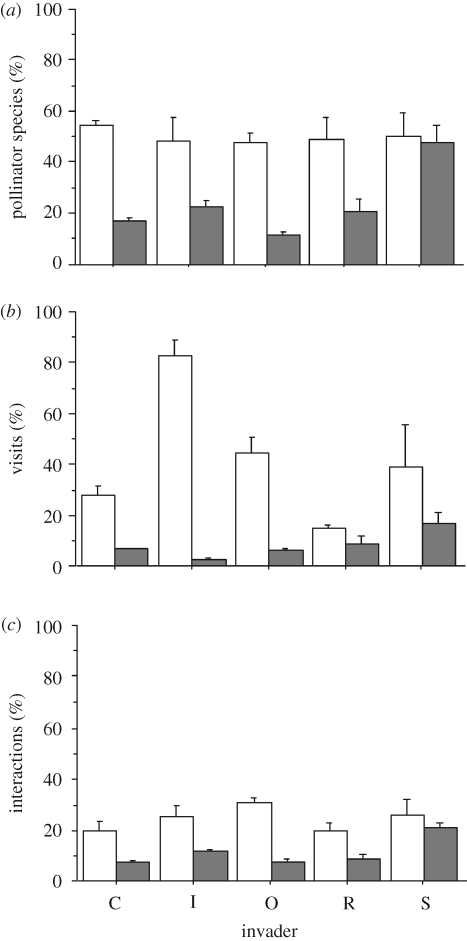
The invader plants were visited by half of all pollinator species occurring in a site, whereas native species received visits from only 18.18 per cent of pollinator species (figure 1a). However, there was no significant change in pollinator richness between invaded and uninvaded networks (sign-rank test, p = 0.99). The invader species contributed to 31.17 per cent of the total visits to the network being visited more than five times than any particular native species (6.48%; figure 1b). In fact, the invader was the most visited species in 60 per cent of the plots. Invaded plots received almost one-third more visits than uninvaded networks (sign-rank test, p = 0.03; table 2) but there was no difference in the total number of visits to native species in invaded and uninvaded networks (sign-rank test, p = 0.12).
Figure 1.
Percentage (+s.e.) of (a) pollinator species richness, (b) visits and (c) interactions in alien (C, Carpobrotus affine acinaciformis; I, I. glandulifera; O, O. stricta; R, R. ponticum; S, S. elaeagnifolium) and native (filled bar) plant species in invaded (unfilled bar) plant–pollinator networks.
Table 2.
Main descriptors of invaded and uninvaded plots for five alien plant–pollinator networks. Minimum and maximum values for three paired communities are indicated. Impatiens and Solanum matrices were too small to calculate nestedness.
| total visits |
plant linkage level |
connectance |
relative nestedness |
|||||
|---|---|---|---|---|---|---|---|---|
| alien species | invaded | uninvaded | invaded | uninvaded | invaded | uninvaded | invaded | uninvaded |
| Carpobrotus | 115–185 | 70–92 | 4.58–7.40 | 3.36–4.22 | 16.52–21.76 | 17.77–20.11 | 0.16–0.26 | 0.10–0.39 |
| Impatiens | 107–193 | 6–55 | 1.00–2.37 | 1.00–1.80 | 23.75–33.33 | 20.83–36.00 | ||
| Opuntia | 77–112 | 63–77 | 3.05–3.89 | 2.50–6.00 | 14.05–16.91 | 13.16–17.78 | 0.36–0.38 | 0.11–0.20 |
| Rhododendron | 479–1178 | 393–1020 | 3.93–8.29 | 4.53–6.31 | 15.09–34.52 | 15.77–17.51 | 0.30–0.41 | 0.26–0.40 |
| Solanum | 148–175 | 80–174 | 2.75–4.25 | 2.50–6.00 | 37.04–47.22 | 27.78–75.00 | ||
The invader received 25.86 per cent of all interactions in the networks (figure 1c), doubling the number of interactions to particular native species (8.93%). In more than 50 per cent of the plots, the number of interactions with the invader was greater than the average for native species in the network, and in 73.3 per cent of the plots, the invader interacted more than any other plant species in the network. However, there were no significant differences in the linkage level of plants between invaded and uninvaded networks (sign-rank test, p = 0.61; table 2), nor were there any significant differences when only considering linkage level of native species in the network (sign-rank test, p > 0.99).
(b). Invader effect on particular native plant species
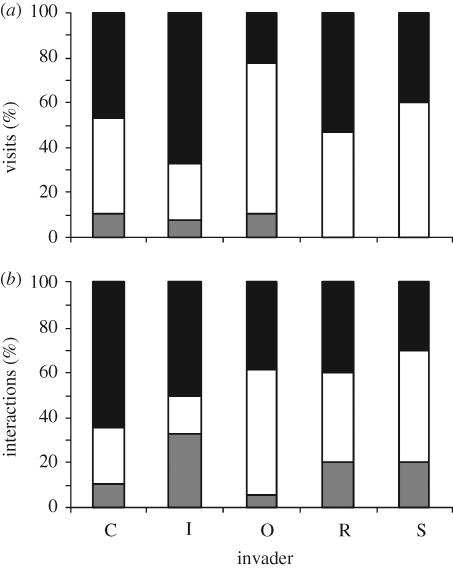
On average, differences in visitation rates and number of interactions between particular native species in invaded and uninvaded plots were not significant (F1,97 = 0.07, p = 0.79 and F1,97 = 1.55, p = 0.22, respectively). That is, there was no clear pattern in whether the effect of the invader on natives was positive, negative or neutral (figure 2). Furthermore, variability in the difference in the number of visits and interactions to particular native species was statistically independent of similarity in flower symmetry (F1,80 = 0.27, p = 0.6 and F1,80 = 0.09, p = 0.77, respectively) and colour (F1,80 = 0.46, p = 0.5 and F1,80 = 1.02, p = 0.32, respectively) between the native and the invader species (results not shown).
Figure 2.
Percentage of pairwise cases with neutral (uninvaded = invaded, grey portion), negative (uninvaded > invaded, negative portion) and positive (uninvaded < invaded, black portion) differences in (a) visitation rates and (b) number of interactions to particular native species when invaded by C, Carpobrotus affine acinaciformis; I, I. glandulifera; O, O. stricta; R, R. ponticum; S, S. elaeagnifolium.
(c). Invader effect on plant–pollinator network structure
Overall, the lack of major changes in the number of interactions with invasion translated to no significant differences in connectance between invaded (20.11%) and uninvaded (21.76%) networks (sign-rank test, p = 0.99; table 2), nor when comparing connectance of native species only (sign-rank test, p > 0.99).
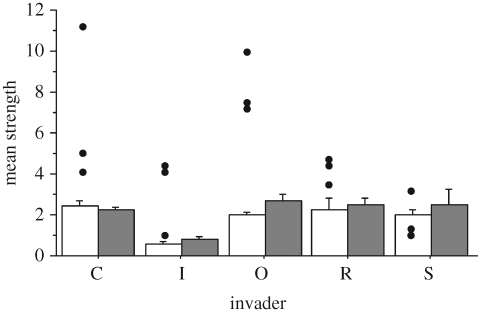
In half of the plots, the strength of the invader was higher than any native plant species in the network (figure 3), and in two-thirds of the plots, it occupied the first quartile position. However, mean strength of native species was not significantly different between invaded and uninvaded plots (sign-rank test, p = 0.302; figure 3).
Figure 3.
Mean strength (+s.e.) of native plant species in uninvaded (filled bar) and invaded (unfilled bar) plant–pollinator networks by C, Carpobrotus affine acinaciformis; I, I. glandulifera; O, O. stricta, R, R. ponticum; S, S. elaeagnifolium. Single dots represent the strength values of the invader plant species in the three sites surveyed.
The plant–pollinator networks were significantly nested in half of the plots when comparing with the benchmark of 1000 random matrices for each plot with null model 2 (data not shown). However, there were no significant differences in matrix nestedness between invaded and uninvaded networks (sign-rank test, p = 0.51; table 2), despite invaders occupying the first position in seven out of nine of the reorganized matrices.
4. Discussion
By comparing paired invaded and uninvaded networks, we have shown that the presence of a single entomophilous alien species in a native plant–pollinator network is associated with consistent integration patterns in the plant–pollinator network. Plant invaders were frequently visited by a large proportion of native pollinator species and constituted a considerable proportion of interactions. Moreover, on average, pollinators depended upon the invader significantly more than on native plant species. In most sites, the invader was the plant species ranking highest in terms of number of interactions with pollinators and dependence of pollinators upon plants. These observations support not only that entomophilous invader species are supergeneralists and are well integrated in the introduced plant–pollinator network (Richardson et al. 2000), but also that they play a central role compared with native plant species that have long evolved with native pollinators (Lopezaraiza-Mikel et al. 2007).
Most visits went to the invader species without a consistent trend on visitation rates and number of interactions to particular native plant species. That is, the presence of the invader had a positive, negative or neutral effect on visitation rates of particular native species. This variability was not associated with differences in flower similarity between the invader and the native species, contradicting the findings of Morales & Traveset (2009) who found a reduction in visits to those native plants that were morphologically similar to the invader. In our case, the native species sample was random, while many previous pairwise studies focused on native species that seemed to be threatened by the alien. Historical factors, as well as other context-dependent factors such as flower abundance and quality and quantity of floral rewards, which are influenced by environmental characteristics, might contribute to the variability of the effect of the invaders on native plant species.
Thus, on average, the high frequency of visits and interactions into the invader did not translate to significant changes in total number of visits and interactions to native plant species in the invaded network. This finding highlights the difficulty of scaling-up the consequences of plant invasion from the effects of pairwise plant-pollination species to community effects (Bjerknes et al. 2007). Furthermore, although the total number of visits to the network increased, the structure of the network did not change with invasion. That connectance and nestedness did not increase with the introduction of a supergeneralist species is somewhat unexpected and shows that the influence of a single alien species in the network, even if it ranks high in the number of interactions it establishes, is buffered by the rearrangement of native plant-pollination interactions after invasion. Whether this result is transient, and might change with time by removing interactions with native plants, remains to be further investigated. Our study networks were small and not highly invaded, a different result might be found in areas with a higher abundance of the invader, where native species have already been displaced and become rare due to abiotic (i.e. nutrients, water, light or space) resource competition. The influence of density-dependent effects on the impacts of invasive plants on the structure of the plant-pollination network deserves further exploration (Muñoz & Cavieres 2008).
In conclusion, we have found that recently introduced, very attractive plant species in a plant–pollinator community reshuffle the number of visits and interactions to native species, but do not change the general architecture of the network that has been exposed to multiple human stresses through millennia. Overall, the structure of plant–pollinator networks is permeable and at the same time robust to the introduction of an alien plant species. An introduced plant becomes as well, or even better, integrated into the plant–pollinator network as coevolved, coexisting native plant species without changing the properties of the plant-community network. Previous evidence of alien species having disruptive effects on plant-pollination interactions are based on highly supergeneralist invasive pollinators and invasion complexes (e.g. Aizen et al. 2008). However, our study shows that the impact of a single alien plant species is probably less disruptive than that of alien pollinators or alien species complexes acting synergically (Simberloff 2006). In the face of increasing invasion rates of plants and pollinators, empirical research is needed to elucidate alien species traits and quantify abundance thresholds which result in disruption of the properties of plant–pollinator networks.
Acknowledgements
We thank C. Zografou for fieldwork assistance and A. Grace for helping with insect taxonomy in Greece. We also thank M.A. Fortuna, J. Bascompte, P. Jordano, L. Harder and two anonymous reviewers for comments on early drafts of this work. Partial research support was provided by the Integrated European Project ALARM—Assessing Large Scale Risks to Biodiversity with Tested Methods (ALARM, http://www.alarmproject.net, contract 506675), the Ministerio de Ciencia e Innovación projects REDESIN (CGL2007-61165/BOS) and Consolider-Ingenio Montes (CSD2008-00040) to M.V., a Science Foundation Ireland Basic Research Grant (04/BR/B0637) to J.C.S. and the E.U.-European Social Fund (80%) and the Greek Ministry of Development-GSRT (20%) to T.T.
References
- Aizen M. A., Morales C. L., Morales J. M.2008Invasive mutualists erode native pollination webs. Plos Biol. 6, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar J. W., Patterson B. D.1995The nestedness temperature calculator: a visual basic program, including 294 presence–absence matrices University Park, NM: AICS Research, Inc. and Chicago: The Field Museum [Google Scholar]
- Bartomeus I., Vilà M.2009Breeding system and pollen limitation in two supergeneralist alien plants invading Mediterranean shrublands. Aust. J. Bot. 57, 109–115 (doi:10.1071/BT08169) [Google Scholar]
- Bartomeus I., Vilà M., Santamaría L.2008Contrasting effects of invasive plant in plant–pollinator networks. Oecologia 155, 761–770 (doi:10.1007/s00442-007-0946-1) [DOI] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Melián C. J., Olesen J.2003The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387 (doi:10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Olesen J. M.2006Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433 (doi:10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- Bjerknes A. L., Totland O., Hegland S. J., Nielsen A.2007Do alien plant invasions really affect pollination success in native plant species? Biol. Conserv. 138, 1–12 (doi:10.1016/j.biocon.2007.04.015) [Google Scholar]
- Blüthgen N., Menzel F., Hovestadt T., Fiala B., Blüthgen N.2007Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346 (doi:10.1016/j.cub.2006.12.039) [DOI] [PubMed] [Google Scholar]
- Buchmann S. L., Cane J. H.1989Bees assess pollen returns while sonicating Solanum flowers. Oecologia 81, 289–294 (doi:10.1007/BF00377073) [DOI] [PubMed] [Google Scholar]
- Fortuna M. A., Bascompte J.2006Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 9, 281–286 (doi:10.1111/j.1461-0248.2005.00868.x) [DOI] [PubMed] [Google Scholar]
- Guimaraes P. R., Jr, Guimaraes P.2006Improving the analyses of nestedness for large sets of matrices. Environ. Model. Softw. 21, 1512–1513 [Google Scholar]
- Lopezaraiza-Mikel M. E., Hayes R. B., Whalley M. R., Memmott J.2007The impact of an alien plant on a native plant–pollinator network: an experimental approach. Ecol. Lett. 10, 539–550 (doi:10.1111/j.1461-0248.2007.01055.x) [DOI] [PubMed] [Google Scholar]
- Memmott J., Waser N. M.2002Integration of alien plants into a native flower–pollinator visitation web. Proc. R. Soc. Lond. B 269, 2395–2399 (doi:10.1098/rspb.2002.2174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J., Waser N. M., Price M. V.2004Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales C. L., Aizen M. A.2002Does invasion of exotic plants promote invasion of exotic flower visitors? A case study from the temperate forests of the southern Andes. Biol. Inv. 4, 87–100 (doi:10.1023/A:1020513012689) [Google Scholar]
- Morales C. L., Traveset A.2009A meta-analysis of impacts of alien vs. native plants on pollinator visitation and reproductive success of co-flowering native plants. Ecol. Lett. 12, 716–728 (doi:10.1111/j.1461-0248.2009.01319.x) [DOI] [PubMed] [Google Scholar]
- Muñoz A. A., Cavieres L. A.2008The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. J. Ecol. 96, 459–467 (doi:10.1111/j.1365-2745.2008.01361.x) [Google Scholar]
- Nielsen A., Bascompte J.2007Ecological networks, nestedness and sampling effort. J. Ecol. 95, 1134–1141 (doi:10.1111/j.1365-2745.2007.01271.x) [Google Scholar]
- Olesen J., Eskildsen L. I., Venkatasami S.2002Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic spear generalists. Divers. Distr. 8, 181–192 (doi:10.1046/j.1472-4642.2002.00148.x) [Google Scholar]
- Ollerton J., Johnson S. D., Cranmer L., Kellie S.2003The pollination ecology of an assemblage of grassland asclepiads in South Africa. Ann. Bot. 92, 807–834 (doi:10.1093/aob/mcg206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanidou T., Potts S. G.2006Mutual use of resources in Mediterranean plant–pollinator communities: how specialized are pollination webs? In Plant–pollinator interactions: from specialization to generalization (eds Waser N., Ollerton J.), pp. 220–244 Chicago, IL: University of Chicago Press [Google Scholar]
- Petanidou T., Kallimanis A. S., Tzanopoulos J., Sgardelis S. P., Pantis J. D.2008Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure, and implications for estimates of specialization. Ecol. Lett. 11, 564–575 (doi:10.1111/j.1461-0248.2008.01170.x) [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008R: a language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing [Google Scholar]
- Rezende E., Lavabre J., Guimaraes P., Jordano P., Bascompte J.2007Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 448, 925–928 (doi:10.1038/nature05956) [DOI] [PubMed] [Google Scholar]
- Richardson D. M., Allsopp N., D'Antonio C. M.2000Plant invasions - the role of mutualisms. Biol. Rev. 75, 65–93 (doi:10.1017/S0006323199005435) [DOI] [PubMed] [Google Scholar]
- Sargent R. D., Ackerly D. D.2008Plant–pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23, 123–130 [DOI] [PubMed] [Google Scholar]
- Selva N., Fortuna M. A.2007The nested structure of a scavenger community. Proc. R. Soc. B 274, 1101–1108 (doi:10.1098/rspb.2006.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff D.2006Invasional meltdown six years later? Important phenomenon, unfortunate metaphor, or both? Ecol. Lett. 9, 912–919 (doi:10.1111/j.1461-0248.2006.00939.x) [DOI] [PubMed] [Google Scholar]
- Stout J. C., Parnell J. A. N., Arroyo J., Crowe T. P.2006Pollination ecology and seed production of Rhododendron ponticum in native and exotic habitats. Biodiv. Conserv. 15, 755–777 [Google Scholar]
- Traveset A., Richardson D. M.2006Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol. 21, 208–216 (doi:10.1016/j.tree.2006.01.006) [DOI] [PubMed] [Google Scholar]
- Valentine D. H.1978The pollination of introduced species, with special reference to the British Isles and the genus Impatiens. In The pollination of flowers by insects (ed. Richards A. J.), pp. 117–123 London, UK: Academic Press [Google Scholar]
- Westphal C., et al. 2008Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 78, 653–671 (doi:10.1890/07-1292.1) [Google Scholar]