Figure 1.
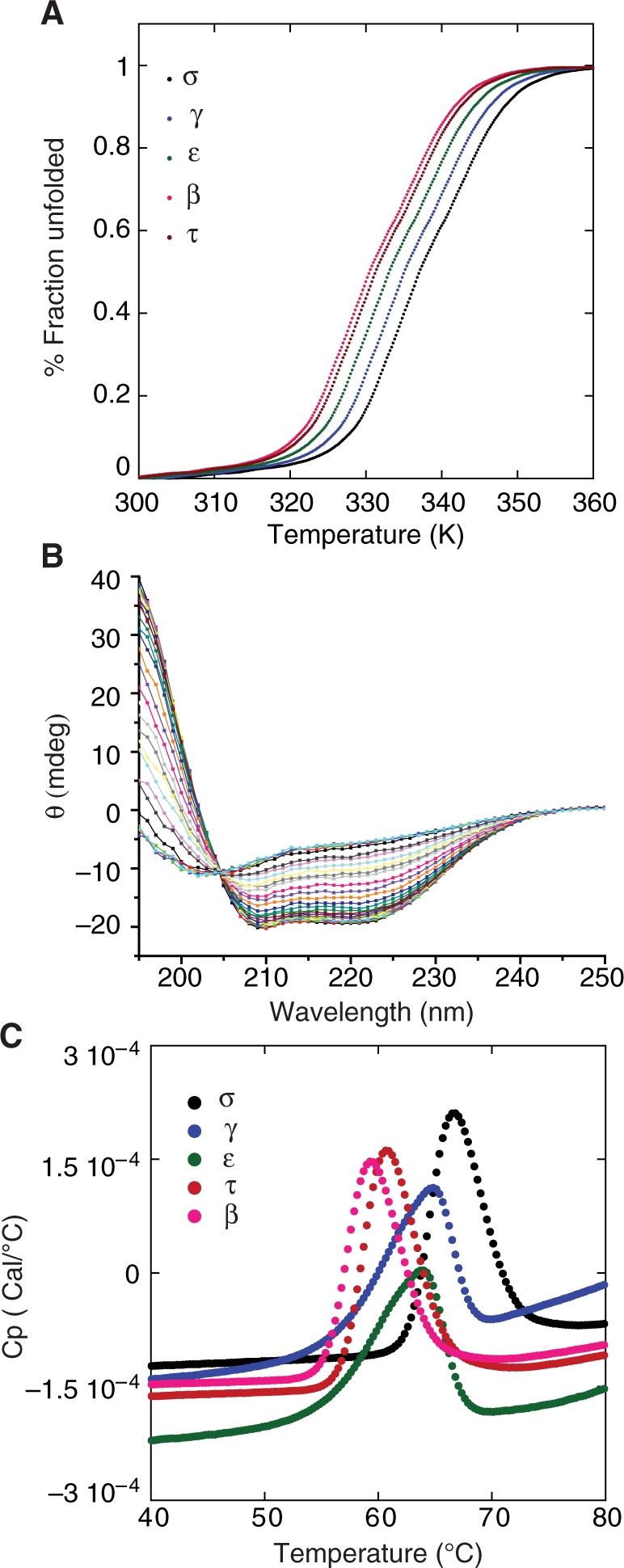
Thermodynamic studies on 14-3-3 isoforms. (A) Thermal unfolding of 14-3-3 isoforms was measured using CD by monitoring the ellipticity at 222 nm. The fraction of unfolded protein is plotted as a function of temperature. Only the σ isoform exhibited reversible folding after denaturation. (B) Isosbestic plot of 14-3-3 σ isoform. Wavelength scans were recorded at different temperatures. A sharp isosbestic point at 205 nm is a clear indication of two-state folding. (C) Thermal denaturation of 14-3-3 isoforms was measured using differential scanning calorimetry in a buffer containing 20 mM HEPES, 150 mM NaCl, 3 mM DTE, pH 7.2 at a heating rate of 250°C/h.
