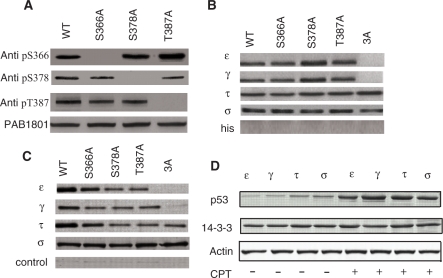
Figure 2.
p53/14-3-3 interaction in vitro and in vivo. (A) Phosphorylation status of p53 upon CPT treatment. Immunoprecipitation with pS366-, pS378- and pT387- specific antibodies indicated that these residues are phosphorylated. (B) Ni pull-down of p53 and its Ala mutants by 14-3-3 isoforms. γ, ε, τ and σ bound single Ala mutants of p53: S366A, S378A and T387A while τ and σ but not γ and ε bound the 3A (S366A/S378A/T387A) mutant. (C) H1299 (p53 null) cells were co-transfected with p53 (and different mutants) and 14-3-3 isoforms in seperate experiment, treated with CPT for 24 h, followed by cell lysis and immunoprecipitated with 14-3-3 isoform-specific antibodies. Control experiments carried out on cells co-transfected with p53 (and mutants) and empty (14-3-3 null) vector showed that the interaction of 14-3-3 with p53 is specific. (D) p53 interaction with 14-3-3 isoforms was significantly enhanced upon CPT treatment. A polyclonal antibody that recognizes all 14-3-3 isoforms (K19, Santa Cruz Biotechnology) and a monoclonal antibody for p53 (PAb1801, Abcam) were used.