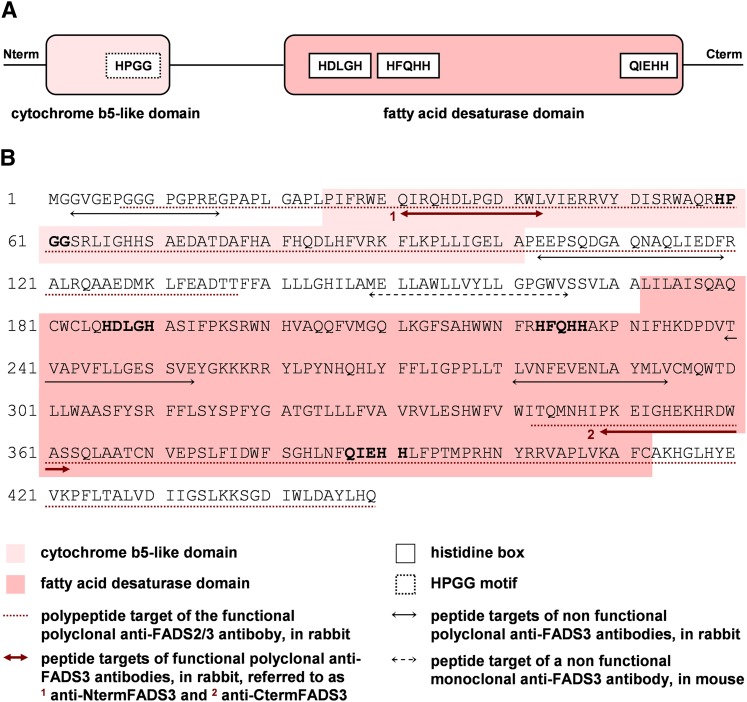
Fig. 1.
Structure of the putative rat FADS3 protein and peptides designed to produce specific rat FADS3 antibodies. A: Rat FADS3 displays an FA membrane-bound desaturase structure with two distinct domains. In the N-terminal end, the cytochrome b5-like domain is specific of front-end desaturases, introducing a double bond before the eighth carbon of the FA carbon chain; the HPGG motif present in that domain is a pivotal pattern for the desaturase activity. In the carboxyl-terminal end, the FA desaturase domain is characterized by three histidine boxes necessary for the catalysis; in the third box, the first amino acid is a glutamine (Q) instead of a histidine (H), which is characteristic of the front-end desaturases such as Δ5-, Δ6-, and Δ8-desaturases, as compared with the methyl-end desaturases like Δ12- and Δ15-desaturases. B: Different antibodies were produced to detect FADS3 in rat tissues. Several peptides were, thus, designed according to the putative sequence of rat FADS3. Among the eight generated antibodies, only three recognized FADS3.