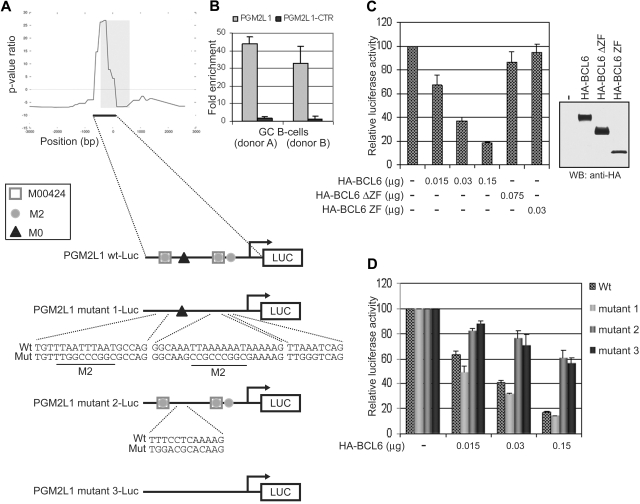
Figure 2.
The M0 site is necessary and sufficient for BCL6 repression on the PGM2L1 promoter. (A) Schematic representation of BCL6 binding to the PGM2L1 promoter as detected by ChIP-on-chip and of luciferase reporter constructs generated to assess the role of M0, M2, and M00424 sites. (B) BCL6 binding to PGM2L1 promoter was confirmed by qChIP assay in GC B cells obtained from 2 donors. A neighboring region (PGM2L1-CTR; −2351/−2546) tested as a control was not enriched. (C) A luciferase reporter construct driven by the PGM2L1 promoter was cotransfected in 293T cells with plasmids expressing HA-BCL6 or its mutants lacking the DNA binding domain (HA-BCL6ΔZF) or the transcriptional repressor domain (HA-BCL6 ZF). The PGM2L1 promoter-driven luciferase construct showed a dose-dependent decreased luciferase activity in the presence of HA-BCL6 but not of its mutants. The relative luciferase activities are displayed as average ± SD of 2 independent transfections. Immunoblotting protein detection of HA-BCL6 wild-type and mutants is displayed on the right. Results are representative of at least 3 independent experiments each performed in duplicate. (D) Disruption of M00424 and M2 sites in PGM2L1 promoter (mutant 1) did not affect repression by BCL6. Conversely, mutations in M0 (mutant 2) were linked to resistance to BCL6 repression, and further mutagenesis of all sites (mutant 3) did not increase the level of luciferase activities. The relative luciferase activities are displayed as average ± SD of 2 independent transfections. Results are representative of at least 3 independent experiments, each performed in duplicate.