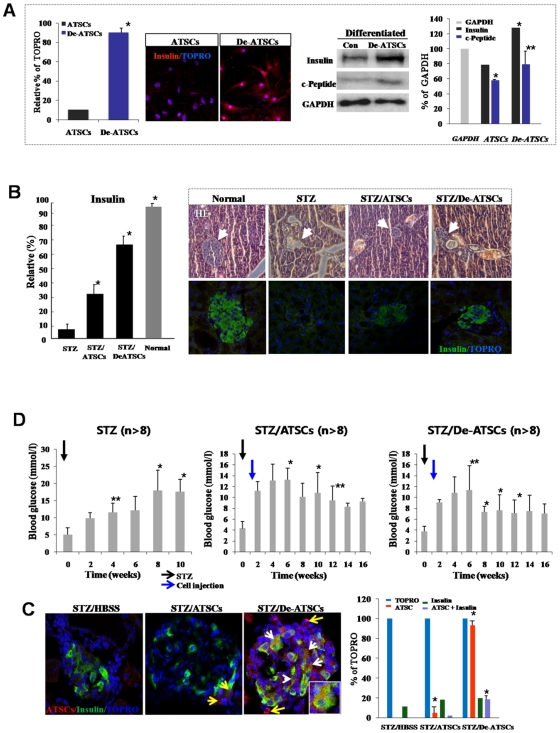
Figure 7. Reversion of the hyperglycemic condition and regeneration of beta-pancreatic islets in de-ATSCs-treated type 1 diabetic mice.
(A) Beta cell differentiation potency of de-ATSCs cells. For beta-like cell differentiation, cells were cultured in “N2 media+NA” containing DMEM/F12. After the induction of differentiation, we conducted immnocytochemistry and western blotting using and insulin and c-peptide antibodies. (B) Quantitative measurement of secreting insulin from de-ATSCs engrafted type1 diabetic animals (left). For analysis of pancreatic islets tissue-derived insulin secreting functions after anti-insulin/green fluorescence marked ATSCs control and de-ATSC cells treatment in diabetes animals (right). Pancreatic islets dissected on day 21 from control and STZ-treated mice were stained with hematoxylin and eosin or (C) immunostained for mouse insulin. Scale bars = 20 μm. White arrows revealed that transdifferentiated and insulin secreting engrafted de-ATSCs. (D) Measurement of blood glucose concentration before and after each cell (2×105 cells) engraftment. Pancreatic damage was induced with intraperitoneal injection of 50 mg/kg of body weight STZ daily for five consecutive days. Datas presented are presented as mean ±SD; n>8. Black and blue arrows appear the date of STZ and cells injection each other. Detail experimental processes was explained in Materials and Methods. Data are represented as mean ±SD; n>8. * p % 0.05, and ** p % 0.01, Student's t test.