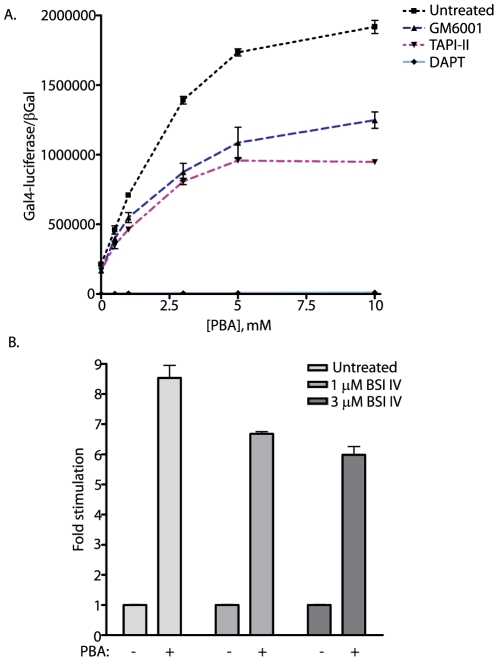
Figure 8. PBA stimulation of APPGV16 requires secretase processing.
In order to determine whether PBA stimulation of APPGV16 is dependent upon proteolytic processing, NAG cells were treated with titrated levels of PBA in the presence or absence of specific secretase inhibitor:. 20 µM GM6001 (broad-spectrum metalloprotease inhibitor), 50 µM TAPI-II (α-secretase inhibitor), or 20 µM DAPT (γ-secretase inhibitor). PBA stimulated a statistically significant 9-fold increase in APP proteolysis (p<0.0001; student t-test). However, co-treatment with GM6001 or TAPI-II decreased the fold-stimulation by approximately half (p<0.0001; two-way ANOVA). DAPT significantly reduced the levels of activity and eliminated the stimulatory effect of PBA (p<0.0001; two-way ANOVA). The β-secretase contribution to PBA enhanced APP proteolysis was examined using two different concentrations of beta-secretase inhibitor (BSI) IV (B). PBA stimulated an 8–9 fold increase in normalized Gal4-luciferase activity. BSI IV decreased Gal4-luciferase activity by less than 25% (p<0.01; student t-test) (B). These data suggest that α-secretase plays a more significant role than β-secretase in PBA enhanced APP proteolysis.