Figure 1.
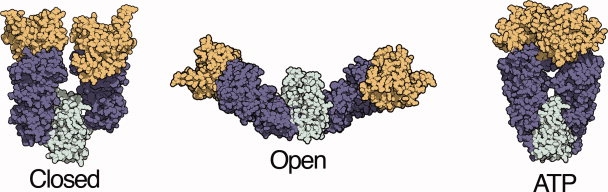
Distinct conformational states of the Hsp90 molecular chaperone. Previous crystallography, electron microscopy, and small angle x-ray scattering (SAXS) measurements have determined a multi-state conformational equilibrium for the Hsp90 homolog from E. coli, HtpG.20–22 The relative population of these conformations can be determined from SAXS measurements and structure-based fitting. The ATP state was modeled from a crystal structure of a yeast Hsp90 homolog.19,20 The closed state is similar to a crystal structure of the Hsp90 homolog from the ER, Grp94, and has been referred to as a Grp94-like state.
