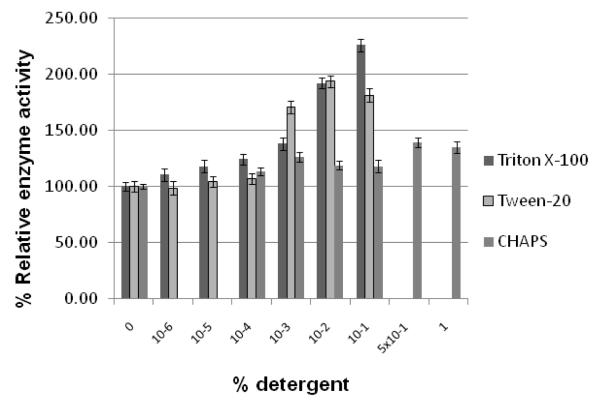
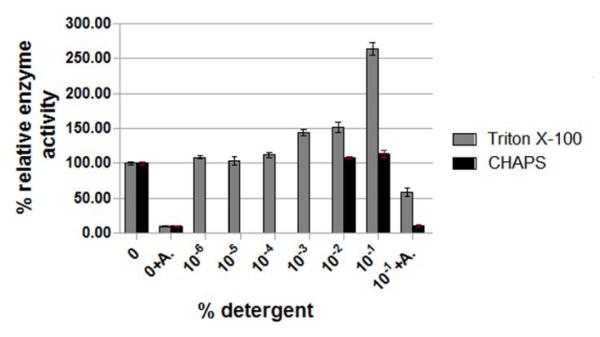
Figure 1. WNV protease activity in presence of different detergents.
Panel A. Standard protease assays were carried out using the tripeptide substrate, t-butyl-oxycarbonyl (Boc)-Gly-Lys-Arg-7-amino-4-methylcoumarin (AMC) as described under Materials and Methods in the absence of any detergent or in the presence of Triton-X100, Tween-20, or CHAPS at the indicated concentrations of 10−6, 10−5, 10−4, 10−3, 10−2, 10−1, or 1 % in the assay mixture.
Panel B. The experimental conditions for the assay were the same as in Panel A except that NP-40, Brij-35, or CHAPS was used at the indicated concentrations.
Panel C. The conditions of the assay were the same except that the effects of Triton X-100 and CHAPS at indicated concentrations on the WNV protease activity using a tetrapeptide substrate, N-Carbobenzyloxy-Val-Lys-Lys-Arg-4-Methoxy-b-naphthylamide as described under Materials and Methods. The percent protease activity was plotted compared to that of the no-detergent control set at 100%.