Abstract
Nicotine, the addictive component of cigarette smoke has profound effects on the brain. Activation of its receptors by nicotine has complex consequences for network activity throughout the brain, potentially contributing to the addictive property of the drug. Nicotinic receptors have been implicated in psychiatric illnesses like schizophrenia and are also neuroprotective, potentially beneficial for neurodegenerative diseases. These effects of nicotine serve to emphasize the multifarious roles the drug, acting through multiple nicotinic acetylcholine receptor subtypes. The findings also remind us of the complexity of signaling mechanisms and stress the risks of unintended consequences of drugs designed to combat nicotine addiction.
Keywords: Nicotine, drug abuse, network, hedonic homeostasis, Calcium
Smoking, perhaps, is the number one preventable cause of serious illnesses like heart disease, stroke and cancer. The fact that nicotine is an addictive drug of abuse is undisputed. At the same time, the drug is thought to be neuroprotective in cases of neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD), as well as in psychiatric disorders like depression and schizophrenia. How the drug mediates its effects is still largely unknown. Here we review what is known about nicotine’s actions on the brain and mechanisms that might contribute to its effects. Using two brain structures, the hippocampus and the Ventral Tegmental area (VTA), known to be involved in some or all effects of nicotine, as examples, we will sequentially address the following issues—a) properties and functional distribution of nicotinic acetylcholine receptors (nAChRs); b) signaling by nAChRs, c) what such mechanisms might inform us regarding nicotine addiction and the role of nAChRs in brain disorders and d) therapeutic approaches to combat these illnesses.
Neuronal Nicotinic Receptors: Subtypes and Distribution
It is well accepted that the actions of nicotine are mediated by its ability to activate neuronal nicotinic receptors (nAChRs). The mammalian nAChR family consists of a number of subunits arising from a total of 11 gene products (α2–α7, α9 α10, β2–β4; Nicke et al. 2004), the α subunits being putative agonist binding subunits, based on homology with the muscle nicotinic receptor (α1). These receptors are arranged as pentamers with the five subunits forming a wall surrounding a central cationic ion channel. Activation of the receptors opens the central pore allowing cations to flux through, depolarizing neurons to their firing threshold.
Distribution of nAChRs in the VTA and hippocampus
In the mammalian brain there are two predominant nAChR subtypes. The first is a homomeric receptor consisting of five α7 subunits (α7-nAChRs) and the second is a heteromer consisting of a combination of α4 and β2 gene products, with or without other subunits (α4β2*-nAChRs; the * reflects the potential heterogeneity in subunits. mRNA for almost all the common brain nAChR subtypes are found in VTA neurons (Charpantier et al. 1998; Champtiaux et al. 2002; Han et al. 2000). There are differences in abundance of message for various nAChR subunits between the hippocampus and the VTA. The mammalian hippocampus appears to contain mainly the α7 mRNAs while the VTA expresses a number of nAChR subunits to varying degrees. In the VTA, there are differences in subunit mRNA abundance between dopaminergic and non-dopaminergic neurons, with the latter mainly expressing α7 mRNAs (Table 1).
Table 1.
Comparison of nAChR subunit mRNA expression in hippocampus and VTA.
| Region | α3 | α4 | α5 | α7 | β2 | β4 |
|---|---|---|---|---|---|---|
| Hippocampus | + | + | + | ++ | + | + |
| Dentate | + | + | + | ++ | + | + |
| Prefrontal | ++ | ++ | +++ | + | ||
| VTA DA neurons | ++ | ++ | ++ | ++ | ++ | ++ |
| VTA Non-DA neurons | + | + | +? | ++ | + | ++ |
Relative distribution of common nicotinic subunit mRNAs in the hippocampus and VTA. (?) indicates conflict among reports. Data compiled from (Court JA et al. 1995; Breese et al. 1997; Rubboli et al. 1994; Ostermann et al. 1995; Lobron et al. 1995; Azam et al. 2003; Azam et al. 2002; Klink et al. 2001).
The two classes of nAChRs are identified by differences in their pharmacology (Table 2). α7-nAChRs have a relatively lower (μM) affinity for nicotine, compared to the heteromeric subtype, while they have a high affinity for the snake venom toxin alpha-bungarotoxin (αBTX). Thus, the distribution of the two receptor subtypes track with high affinity nicotine and αBTX binding (Seguela et al. 1993; Breese et al. 1997; Sorenson and Chiappinelli, 1992). As expected from mRNA distributions, nAChRs are widely distributed in the hippocampus and along the mesolimbic dopaminergic pathway. All dopaminergic neurons at the VTA appear to express a number of pharmacologically identified nAChRs, though only half of these neurons seem to possess the α7 subclass (Klink et al. 2001). Local GABAergic interneurons possess α4β2*-nAChRs (Klink et al. 2001). In the hippocampus, however, α7-nAChRs predominate in the stratum radiatum interneurons (Klein and Yakel, 2005) while heteromeric subtypes might exist in interneurons from other regions, e.g. stratum oriens (Khiroug et al. 2004). Using radiolabeled methyllycaconitine (MLA) an antagonist selective for the α7-nAChRs (Ward et al. 1990) and nicotine binding studies to discriminate between α7-nAChRs and α4β2*-nAChRs, it was shown that the hippocampus contained approximately two-fold greater α7-nAChR sites than the VTA but about 4-fold less of the α4β2*-nAChR sites (Mugnaini et al. 2002).
Table 2.
Functional nAChR pharmacology.
| Subtype | Agonist | EC50 (μM) | Antagonists | IC50 (μM) |
|---|---|---|---|---|
| α7-nAChRs | ACh | 1301, 1552 | αBTX | 0.00165 |
| Nicotine | 492, 273 | MLA | 0.000256 | |
| Choline | 16001, 4934 | Conotoxin ImI | 0.0227 | |
| Mecamylamine | 15.68 | |||
| α4β2*-nAChRs | ACh | 2.13 | DHβE | 0.0810 |
| Nicotine | 0.99 | Mecamylamine | 0.77–2.38 |
Data compiled from 1) Alkondon et al. 1997; 2) Gopalakrishnan et al. 1995; 3) Alkondon and Albuquerque, 1993; 4) Gonzalez-Rubio et al. 2006; 5) Zhang et al. 1994 6) Palma et al. 1996; 7) McIntosh et al. 1999; 8) Papke et al. 2008; 9) Alkondon and Albuquerque, 1995; 10) Buisson et al. 1996.
Subcellular distribution
In neurons, functional nAChRs are not only widely distributed across different neuron types but are also differentially localized within a neuron—on the soma, dendrites or synaptic terminals. This diverse distribution of the receptors presents a challenge to the functional interpretation of receptor activation. It suggests a modulation of plasticity and activity at a network level more than linear modulations of individual synaptic pathways. In this section, we summarize evidence for functional localization of nAChRs.
Presynaptic terminals
nAChRs are present at presynaptic terminals of other transmitter systems where they modulate transmitter release. The key subtype mediating these effects is the α7-nAChR while other subtypes play a role as well. Together these receptors at presynaptic locations influence the release of glutamate (Gray et al. 1996; Sharma et al. 2008; Sharma and Vijayaraghavan, 2003), GABA (Lena and Changeux, 1997), norepinephrine (Clarke and Reuben, 1996), and dopamine (Grady et al. 2007), among others. Modulation of transmitter release at various nerve terminals could potentially be a mechanism for nicotine-mediated alterations in local synaptic plasticity. Small, spontaneous release events, increased by nicotine, can modulate local protein synthesis and affect synaptic efficacy (Sutton et al. 2004). At the mossy fiber terminals of the rat hippocampus, nicotine mediates an unusual form of plasticity. The drug causes a burst of glutamate release events in the absence of incoming action potentials, mediated by α7-nAChRs (Sharma and Vijayaraghavan, 2003). This burst consists of both an increase in glutamate release frequencies as well as a CaMKII-mediated concerted release of multiple quanta at these terminals (Sharma et al. 2008). Activation of α7-nAChRs induces a long-lasting slow calcium transient at these terminals resulting, at least partially, from the release of calcium from internals endoplasmic reticulum stores (Sharma et al. 2008). Most surprisingly, this burst of transmitter release is sufficient to drive the postsynaptic neuron above its firing threshold, in what is the first instance of a presynaptic action potential-independent transmission in the CNS (Sharma et al. 2008; Sharma and Vijayaraghavan, 2003). The relevance for this signaling mechanism for nicotine actions in vivo comes from the observation that at physiological temperatures, levels of nicotine found in the serum of smokers can mediate these effects (Sharma et al. 2008). The findings suggests that, at this synapse, nicotine ‘hijacks’ normal signaling pathways, altering synaptic strength in a manner independent of the physiological context but dependent on the presence of nicotine. Such modulation might have a very significant impact nicotine-mediated addictive processes and would be consistent with the more homeostatic view of addiction discussed below.
GABA interneurons
A common site for functional nAChRs appears to be at the soma and dendrites of GABAergic interneurons. In the VTA, local GABAergic neurons, as well as the feedback GABAergic input from the nucleus accumbens, contain α4β2*-nAChRs (Fagen et al. 2003; Mansvelder et al. 2003). These receptors, upon activation of cholinergic inputs might regulate the firing of the VTA output neurons controlling the patterning and timing of responses.
In the hippocampus, interneurons of the stratum radiatum, and other regions, contain α7-nAChRs. The receptors appear to be localized both at somatic and dendritic sites (Fayuk and Yakel, 2007; Klein and Yakel, 2006; Klein and Yakel, 2005) as well as at interneuron terminals where they modulate GABA release (Alkondon et al. 1999). However, α7-nAChRs are also present on secondary GABAergic interneurons and can thus mediate both inhibition as well as disinhibition of the output pyramidal neurons (Ji and Dani, 2000).
Principal neurons
There is evidence for the existence of functional nAChRs on dopaminergic output neurons at the VTA (Mansvelder et al. 2003). Putative α4β2*-nAChRs at somatodendritic sites might provide excitatory inputs for these neurons while α7-nAChRs at nerve terminals would enhance dopamine release at the nucleus accumbens, the target area. Coupled with the modulation of interneurons by nAChRs, these results suggest a complex regulation of the reward pathway by nAChRs.
In the hippocampus, evidence for the existence of nAChRs on the pyramidal neurons, the principal excitatory output neurons of the area, is more equivocal. While small nicotinic currents have been demonstrated from these neurons (Ji et al. 2001), other studies have failed to discern these signals (Khiroug et al. 2003).
Astrocytes
Studies over the last decade have demonstrated that astrocytes have more active role to play in modulation of synaptic activity than previously imagined. These cells have a form of excitability, not mediated by fast action potentials, but by slowly propagating calcium signals (Araque et al. 1999). In addition, accumulated evidence suggests an ability of these cells to communicate back to neurons by release of multiple vesicular and non-vesicular signals (Haydon, 2001).
Our studies showed that hippocampal astrocytes have functional α7-nAChRs that induce large calcium transients in these cells (Sharma and Vijayaraghavan, 2001). These signals result from a complex cascade of amplification upon receptor activation, discussed below. Presence of nAChRs on astrocytes in situ has been demonstrated as well (Gahring et al. 2005; Gahring et al. 2004a; Gahring et al. 2004b), suggesting another site for nicotinic modulation in the brain. As astrocytes are thought to be involved in controlling the local excitation of neuronal synapses, nicotinic control of these cells might contribute to the overall effects of the drug on synaptic function and plasticity.
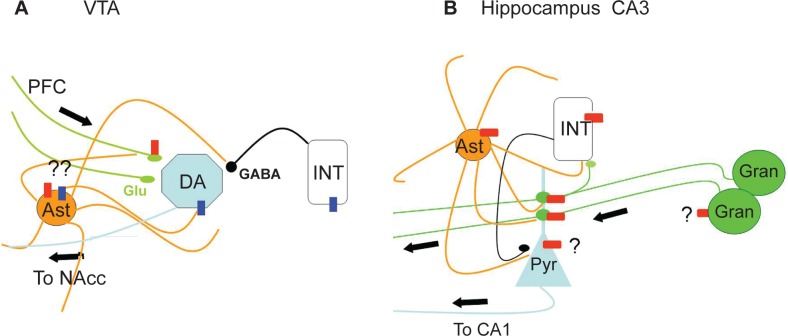
In summary, functional nAChRs are widespread in their distribution among various cell types in the brain, controlling all aspects of signaling between neurons. This potentially complex modulation of network activity by nAChRs is illustrated in Figure 1. These findings suggest caution in linear interpretations of the drug effects in order to arrive at mechanistic correlates of behavioral changes induced by the drug.
Figure 1.
Intra-circuit distribution of functional nAChRs. A) α7-nAChRs (Red Boxes) and α4β2-nAChRs (Blue Boxes) distribution in the VTA. The homomeric receptors are predominant on the glutamatergic terminals of the inputs from the prefrontal cortex (PFC; Green lines). The heteromeric receptor is present on the GABAergic interneurons (INT) and on the Principal dopaminergic neurons (DA) which send their outputs to the nucleur accumbens (NAcc). The presence of functional nAChRs on astrocytes (Ast) from the VTA has yet to be demonstrated (denoted by ?). Based on data from other systems, however, this is a distinct possibility. B) Distribution in the CA3 region of the hippocampus. The main functional evidence available is for the α7-nAChRs (Red Boxes). The homomeric receptor is on the GABAergic interneurons (INT) and at the mossy fiber boutons (Green circles) that are en passant terminals made on to the dendrites of the CA3 pyramidal cell (Pyr) and originating from the granule cells (Gran) of the dentate gyrus. Hippocampal astrocytes (Ast) possess functional α7-nAChRs. The presence of functional nAChRs on granule cells and the pyramidal cells are controversial (indicated by ?). The nAChR modulated output is the axons of the CA3 pyramidal cells that innervate the Principal cells in the CA1 region. The figure illustrates the potentially complex modulation of network output by nAChRs.
nAChR Signaling Mechanisms
The widespread incidence of nAChR-mediated rapid chemical transmission has yet to be demonstrated in the brain. In the stratum radiatum interneurons of the mammalian hippocampus, α7-nAChR synaptic currents were reported after the blockade of other major transmitter systems with antagonists (Frazier et al. 1998) while these were not observed in another study (McQuiston and Madison, 1999). Similarly, a fraction of synaptic currents recorded from CA1 pyramidal cells were consistent with nAChR responses (Hefft et al. 1999). However, a number of caveats plague these studies, the chief being the lack of information on the relationships between nAChRs, acetylcholine esterases (AChEs), and cholinergic innervation in an acute slice preparation, as well as an over reliance on pharmacological agents.
Both the unusual locations of these receptors, as well as the observations that very few postsynaptic specializations have been observed at ACh release sites (Contant et al. 1996), suggest a nontraditional role for nAChR activation, probably by transmitter diffusion.
Such an idea runs into the problem of having to account for receptor desensitization. All ligand-gated ion channels desensitize, some faster than others. Among nAChRs, the α7-nAChR subtype shows rapid desensitization with nicotine with a time constant of a few milliseconds, while the slowly desensitizing receptor types do so in the order of a few seconds (Zhang et al. 1994). It has been argued that at low doses of nicotine might desensitize the α4β2*-nAChRs but not the α7-nAChRs due to the fact that the latter has lower affinity for nicotine (Fenster et al. 1997; Mansvelder et al. 2003). While one cannot rule out the role of desensitization in mediating the behavioral effects of nicotine, the direct implication of this channel property might be difficult to demonstrate. First, channel kinetics and biophysical states of these receptors are not completely understood. Second, the assumption underlying these arguments, that nicotine concentrations seen in the serum of smokers are the same as that encountered by the receptors at synaptic and extrasynaptic sites, is not necessarily correct. In fact, nicotine might be present at higher concentrations in the brain and might have slower clearance rates (Ghosheh et al. 2001). Third, effects attributed to receptor desensitization might be effects downstream to receptor activation, e.g. presynaptic alterations in glutamate release probabilities in areas where nAChRs modulate its release. Lastly, the relationship between time courses of desensitization and inactivation (which are often used synonymously in nAChR literature) and nicotine-mediated changes in behavior are not intuitively obvious. The diffuse distribution of nAChRs makes it difficult to predict what the consequences of either activation or desensitization might be for network excitability and plasticity. In physiological context, it is also not obvious how channel desensitization would affect signaling based on ACh diffusion.
A unique feature of nAChRs, especially the α7-nAChR subtype, is that they have a high relative permeability for calcium and can also effectively raise intracellular free calcium concentration ([Ca]i). This ability of the receptors arises from being coupled to downstream calcium amplification mechanisms. Studies indicate that calcium flux through α7-nAChRs can be dramatically amplified by downstream release of calcium from ER stores via CICR (Sharma et al. 2008; Sharma and Vijayaraghavan, 2003; Vijayaraghavan et al. 1992). This ability of α7-nAChR to efficiently raise intracellular calcium levels plays a dominant role in the physiological function of the receptors and makes them effective mediators of downstream calcium signaling cascades (McKay et al. 2007; Dajas-Bailador and Wonnacott, 2004). The α4β2-nAChRs, on the other hand may play a more traditional role for ligand-gated ion channels which is to provide the initial depolarization for neuronal firing. At the same time, by activating voltage-gated calcium channels (VGCCs), these receptors would also play important roles in nAChR-mediated calcium signaling.
The time course of calcium signals generated by nAChRs as well as consequent physiological responses is also not consistent with receptor desensitization. In response to nicotine application, α7-nAChRs on mossy fiber terminals in the hippocampus show slowly rising calcium transients with decay times dependent on the duration of agonist application in the order of many seconds (Sharma et al. 2008). While most of the calcium signals observed in response to α7-nAChR activation comes from amplification via CICR, it still does not explain agonist exposure time-dependent signals, over periods up to 200s generated by a receptor that desensitizes in milliseconds (Sharma et al. 2008; Sharma and Vijayaraghavan, 2003). These results, coupled with many studies on the downstream effects of α7-nAChRs (e.g. Berger et al. 1998; Dajas-Bailador et al. 2000) suggest the possibility that a small, slow desensitizing component might be the relevant α7-nAChR signal mediating extrasynaptic and calcium-dependent effects of the receptor. If true, this would imply that measuring calcium signals might be a more sensitive assay for functional α7-nAChRs than whole cell current measurements. The idea remains to be tested.
Considering nAChRs primarily as modulators of calcium signaling rather than primary mediators of synaptic transmission makes the idea of volume transmission more feasible as they might not require fast and efficient delivery of ACh. Evidence for such a mechanism must, however, come from further studies on the nature of cholinergic signaling. For example, determining the extent of agonist diffusion requires adequate knowledge of the relative distribution of nAChRs, transmitter release sites, and local AChE concentrations. It is tempting to speculate that, unlike the neuromuscular junction, the AChE forms a pocket around release sites providing a reasonable distance for free diffusion of ACh enabling it to act on nAChRs within the pocket. This idea needs to be tested.
Nicotinic Receptors in Disease States
Autosomal dominant frontal lobe epilepsy (ADNFLE)
Some information on what role these receptors might play in CNS physiology comes from examining phenotypes of naturally occurring mutations in nAChRs genes. ADNFLE has been shown to be linked to nAChRs (Marini and Guerrini, 2007) which includes the α4 gene mutations (Phillips et al. 2000) as well as mutations in the β2 gene (Bertrand et al. 2005; Phillips et al. 2001). In vitro studies, using receptors reconstituted in Xenopus oocytes, have shown that relevant mutations to the α4 subunit results in a receptor response that shows increased desensitization and altered calcium permeability (Bertrand et al. 1998). In mice harboring the mutation, exposure to nicotine elicits a behavior termed as the dystonic arousal complex, a collection of symptoms akin to those seen in ADNFLE (Teper et al. 2007). Similarly, ADNFLE mouse models show an increased sensitivity for the seizure generating effects of nicotine (Klaassen et al. 2006). While these studies cannot be over generalized as nAChR mutations might account for only a subpopulation of ADNFLE sufferers, the results nonetheless demonstrate a correlation between altered nAChR activity and changes in neuronal excitability. Interestingly, many pathological nAChR mutations can be localized to regions predicted to affect ligand-induced channel gating from structural models (Taly et al. 2006).
α7-nAChRs in schizophrenia
Schizophrenic patients show a much greater incidence of smoking than the general population (e.g. see Gopalaswamy and Morgan, 1986). These findings led to the examination of nAChRs in schizophrenia. (Bickford et al. 1993).
Auditory gating is measured as changes in a specific peak in EEG recordings. This response, known as the P50 auditory evoked response, is seen about 40–80 ms after the presentation of the auditory stimulus. In normal population presentation of two stimuli closely spaced in time (~500 ms) results in the attenuation of the P50 response to the second stimulus. This relative suppression of the P50 response is an indicator of sensory gating. There is much less suppression of the P50 evoked response in schizophrenics (Cullum et al. 1993), leading to the idea that defects in this process contribute to schizophrenic symptoms. Consistent with epidemiological data, smoking restores, to a large extent, the P50 ratios in schizophrenics (Adler et al. 1993) implying a role for nAChRs in this process. A number of studies indicate that P50 deficits show significant correlation with the level of α7-nAChRs in the brain (Adler et al. 1998). Further, infusion of α7-nAChR antagonists decrease P50 ratios (i.e. response to the second tone not suppressed) while agonists increase them (Simosky et al. 2003). This effect is mimicked by the atypical antipsychotic, clozapine, in a manner consistent with its effects being via α7-nAChRs (Simosky et al. 2003). Linkage analyses showed that the P50 changes were mapped to the chromosomal locus 15q13–q14 (Leonard et al. 2000). The α7 gene lies within this locus thus providing good correlation between α7-nAChRs, P50 deficits and schizophrenia (Freedman et al. 1997). These results also suggest that therapeutic interventions based on modulating α7-nAChR function might be useful in the treatment of certain schizophrenic symptoms as well.
Nicotine and Alzheimer’s disease
A key finding over the years has been the role of α7-nAChRs in neuronal survival (Mechawar et al. 2004; Berger et al. 1998; Roy et al. 1998; Pugh and Margiotta, 2000; Dajas-Bailador et al. 2000) increasing the plausibility of a role for these receptors in neurodegenerative diseases.
Nicotine has been shown to increase memory and attention in normal humans (Warburton, 1992). In AD the drug has been shown to improve memory deficits. This idea is supported by epidemiological data suggesting that incidence of AD among smokers is significantly less than in non-smokers (Perry et al. 1999). The cholinergic hypothesis for AD has been prevalent for a long time based on the finding that loss of basal forebrain cholinergic neurons is one of the early symptoms of AD. This led to the use of acetylcholine esterase (AChE) inhibitors for treatment of the disease (Bartus et al. 1982). The results from this line of therapy have been disappointing, thus undermining the idea as a whole. Upon reflection, however, these findings are not contradictory to the cholinergic hypothesis. The efficacy of the AChE inhibitors, whose function is to increase the lifetime of the transmitter in the extracellular space, depends on the presence of cholinergic projections. If these are the earliest neurons to die, as suggested, the loss of projections to would render inhibition of AChE ineffective. More recent data suggests that nAChR agonists and antagonists might be a better, more effective, therapeutic approach to the disease. Some attempts at drug development based on this idea have been made. This is summarized in the next section.
A feature of α7-nAChRs is their modulation by the beta amyloid 1–42 peptide (Aβ), the key component of plaques found in the brain of AD patients. Aβ binds to the receptor with picomolar affinity (Wang et al. 2000) and it has been shown that the peptide, by activating α7-nAChRs, can modulate the MAPK pathway and CREB activation (Dineley et al. 2001). At the same time the receptor is down-regulated in AD brains (Oddo and LaFerla, 2006). In transgenic mouse models of AD, nicotine via the MAPK pathway increases the hyperphosphorylation of the tau protein, the cause of neurofibrillary tangles, which is a part of the AD pathology as well (Oddo and LaFerla, 2006; Oddo et al. 2005). Thus, the exact mechanisms which mediate the enhanced cognitive functions seen upon nicotine treatment remains unclear.
Parkinson’s disease
Nicotine has been shown to be protective against PD as well (for a recent review see Singh et al. 2007). In PD, there is a specific loss of the dopaminergic neurons of substantia nigra, which provide inhibitory control to the neurons of the striatum. Once again the role of nAChRs is likely to be complex, involving differential modulation of a number of pathways. In mouse models of PD, where selective lesions of dopaminergic neurons were made by injection of 6-hydroxy dopamine (6-OHDA) selectively into the striatum or the substantia nigra, the levels of a number of nAChRs showed dramatic decline though the α7-nAChR levels remained unchanged (Jellinger, 2002). In patients with PD there is a selective increase in α7-nAChRs while levels of heteromeric nAChRs decline (Bordia et al. 2007; Janhunen and Ahtee, 2007). α7-nAChRs have been shown to trigger an anti-inflammatory pathway in brain microglia (Shytle et al. 2004). The activation of the receptor can suppress the inhibition of pro-inflammatory transcription factors NFkappaB and c-myc (Liu et al. 2007).
In neurons, dopamine is oxidized by monoamine oxidases (MAOs). One class of MAO; MAO-B, oxidizes dopamine and various primary and tertiary amines to their corresponding aldehyde and free amines, resulting in the release of hydrogen peroxide a source of free radicals. The oxidation of dopamine to dihydroxy phenyl acetic acid, via a series of reactions, generates a number of reactive oxygen species (ROS). As the brain has a more limited capacity to clear ROS than other tissues, these species can trigger a cytotoxic cycle, wherein in the presence of solvated Fe(II) and H2O2, the toxic 6-OHDA is formed (Fenton Reaction). The 6-OHDA, in turn, is able to mobilize more Fe(II) from stored forms of iron in proteins, thus propagating neurotoxicity and neuronal death. Nicotine has been shown to be neuroprotective by blocking MAO activity, thus acting as a protective antioxidant (Linert et al. 1999). This is discussed further under the section on clinical options (see below).
Thus there are both epidemiological, as well as potential mechanistic bases, for the protective role of nAChRs in neurodegenerative diseases and it is likely that α7-nAChRs play an important role.
Nicotinic Receptors and Addiction
Nicotine and smoking
The peak concentrations of nicotine reached in the bloodstream upon smoking a cigarette is ~0.5 μM–1 μM (Benowitz and Henningfield, 1994; Henningfield et al. 1993). However, nicotine appears to be concentrated in different compartments. In the brain, the concentrations of nicotine might be as much as five-fold that of the serum (Ghosheh et al. 2001). As the substance is hydrophobic and can also enter cells, it is almost impossible to determine concentrations at synapses. This becomes an interpretational problem, as discussed below.
Potential mechanisms underlying nicotine addiction
The fact that nicotine is an addictive drug is no longer in dispute, if it ever was. On the other hand, the mechanistic basis of this process is still far from clear.
A large body of literature has focused on the role of the mesolimbic dopaminergic circuit in addiction, linking addiction to reward seeking behavior. In 1958, James Olds published a series of papers identifying this system as the ‘pleasure centers’ of the brain. These studies demonstrated that electrical stimulation of these areas resulted in a powerful positive drive in animals that proved stronger than other innate drives like hunger and self-preservation. Further, placing electrodes in this region resulted in a strong self-stimulation by the animals (OLDS, 1958b; OLDS, 1958a; OLDS, and OLDS, 1958; OLDS, 1958c). The link between these studies an addiction, however, came a couple of decades later, with studies demonstrating that delivering drugs of abuse, like cocaine, at the reward centers identified by Olds resulted in the same behavior resulting in an equally strong drive to self-administer these agents (Goeders and Smith, 1983). The findings resulted in a paradigm shift in our view of addiction changing from a negative drive, i.e. to avoid withdrawal symptoms, to a positive connotation to the drugs themselves.
In the subsequent years, a vast literature was generated showing the action of various drugs on the mesolimbic reward system (McClung and Nestler, 2008; Koob and Nestler, 1997; Nestler, 1994a; Nestler, 1994b; Nestler et al. 1993; Nestler, 1992).
The mesolimbic reward system originates as a bundle of dopaminergic fibers at the ventral tegmental area (VTA) and fans out to a number of limbic areas including the nucleus accumbens (NAcc) and the prefrontal cortex. Dopaminergic neurons of the VTA are under the control of both GABAergic interneurons as well as incoming glutamatergic inputs from the prefrontal cortex. These glutamatergic inputs into the VTA provide the main excitatory control for dopamine release to further downstream areas of the pathway (Sesack and Pickel, 1992; Taber et al. 1995; Taber and Fibiger, 1995). Some of these inputs also terminate at the nucleus accumbens potentially providing a positive feed forward mechanism at the reward pathways (Sesack and Pickel, 1992).
There appear to be two pathways by which drugs of abuse modulate the mesolimbic dopaminergic system: the first is the direct alteration of signaling at the VTA and the second is the modulation of dopamine release at terminals in the NAcc. Nicotine appears to modulate both the excitatory as well as inhibitory inputs on the dopaminergic neurons of the VTA.
Drug Addiction: A homeostatic view
The prevailing wisdom is that addiction is a learnt behavior, which implies that like other forms of learning and memory, the consequence of nicotine exposure would be a net increase in long-term synaptic strength. It is being recognized that widespread distribution of receptors for drugs of abuse implies that their action is not restricted to the mesolimbic reward pathway. This leads to a paradigm shift in our way of thinking about addiction and other actions of these drugs. nAChRs are present in a number of cortical, midbrain, and hindbrain loci.
An attractive concept put forth a few years ago by Koob and colleagues. The idea is that drug addiction is a means of self-medication for maintaining hedonic homeostasis in the brain- an acceptable balance of positive and negative affective states. According to this theory, drug addiction is a complex phenomenon involving a lot more than just activation of reward pathways. It also links the addictive process to emotional dissonance explaining the greater prevalence of drug abuse among people with mood disorders. This, more inclusive, viewpoint most certainly involves a host of neurotransmitter systems in a number of areas of the brain.
The term ‘allostasis’ is used to refer to this condition where the brain must vary all its parameters to match them to perceived environmental demands, in order to maintain stability. This also implies that when stability is achieved, it includes the additional external variable, the drug, and withdrawal of the drug would consequently lead to instability. One consequence of this instability in addicts would be a negative hedonic balance, leading to craving for the drug, attentional bias in the form of preoccupation and anticipation, all of which lead to continued drug use or relapse (Koob and Le Moal, 1997; Koob, 1996). Changes in synaptic strength induced by previous exposure to addictive drugs would now lead to spiraling distress as the brain attempts to reach a new homeostasis.
There are a number of attractive facets to this idea. First, it implies that a vast number of experiences, drug related or not, can be addictive, a conclusion intuitively attractive. Second, it takes into account the non-linearity of signal processing in the brain, raising the idea that it is possible to arrive at a common endpoint from a number of different circumstances. Such a mindset would also be valid for other psychiatric disorders. Third, it allows for a common perspective for the action of a number of addictive drugs that have a wide range of targets and end results and also provides an easier explanation for the small degree of co-morbidity among drugs of abuse. Fourth, as the brain will go into a long lasting dynamic instability until it finds a new, acceptable, homeostatic state, it also predicts the possibility of relapse after considerable periods of abstinence. Lastly, it recognizes the importance of the individual in the addiction process, implying that the strength and nature of the drugs abused would depend on the initial and the end hedonic state of the individual. It drives addiction research from searching for common brain pathways to more individually tailored hypotheses, implying that it might be futile to look for a one size fits all treatment approaches. This is definitely borne out in clinical studies with various treatment options to tobacco addiction.
The wide distribution of nAChRs is certainly consistent with this view. Our recent findings that activation of α7-nAChRs in the hippocampus leads to synaptic transmission independent of information coming down the presynaptic axon (Sharma et al. 2008; Sharma and Vijayaraghavan, 2003). This is consistent with the idea of allostasis in that nicotine usurps normal signaling pathways resulting in strengthening of synapses in non-physiological contexts. Such an altered homeostatic state is now dependent on the presence of the drug, the withdrawal of which would result in instability and distress.
Current Treatment Options
A number of treatment options are currently available or in the development process to target nicotine addiction. In this section, we will briefly summarize some of the more promising approaches that can augment, or substitute for, current nicotine replacement therapies (Table 3).
Table 3.
Potential therapeutic compounds targeting nAChRs.
| Drug | Target | Postulated effects | Potential use |
|---|---|---|---|
| Nicotine Vaccine | Serum Nicotine | Blocking drug entry into the brain | Smoking cessation |
| Varenicline | α4β2*- and α7-nAChrs | Affects mesolimbic dopamine levels | Smoking cessation |
| GTS-21 and 4OH GTS-21 | α7-nAChRs | Improves auditory gating | Schizophrenia |
| Tropisetron | α7-nAChRs | Improves auditory gating | Schizophrenia |
| TC-1698 | α7-nAChRs | Neuroprotection | AD and PD |
| Methoxsalen | CYP2A6 inhibitor | Alter nicotine clearance | Smoking cessation |
| Selegiline | CYP2A6 blocker, MAO inhibitor | Affects nicotine clearance, dopamine metabolism | Smoking cessation, PD |
| Moclobemide | MAO inhibitor | Alters Dopamine metabolism | Smoking cessation, Depression |
| Buproprion | Dopamine Transporter Blocker | Affects mesolimbic dopamine levels | Smoking cessation and Depression |
| Nortriptyline | NE/5HT transporter blocker | Increases brain NE and serotonin levels | Smoking cessation and Depression |
| Naltrexone | μ-opioid antagonist and α7-nAChR channel blocker | Anxiolytic and antidepressant | Smoking Cessation |
Nicotine Vaccines
A recent approach to tackling nicotine addiction is to raise antibodies against the drug to prevent its access to the brain. A number of companies are developing such vaccines (Novartis, Sanofi-Aventis, Nabi Biopharmaceuticals, Xenova Group). The idea is to link nicotine, as a hapten, to carrier proteins like the bacteriophage Qβ coat protein (Cornuz et al. 2008), in order to make it an effective immunogen.
While initial studies have shown this approach to result in a statistically significant increase in the rates of abstinence among smokers, the long-term efficacy of this approach remains to be determined. While no serious side effects of this treatment have been noted in humans, a number of issues need to be resolved and await a longer-term examination of this approach (LeSage et al. 2006b; LeSage et al. 2006a). A risk, is that, as the vaccine might only sequester a fraction of the serum nicotine, it might induce patients to smoke more. While this concern has not been borne out from limited studies, it still needs to be resolved. A second issue is one of withdrawal. As would be expected, in the presence of nicotine antibodies, the drug does not alleviate its own withdrawal symptoms. An interpretation of this outcome would be that nicotine use would no longer be rewarding to ameliorate withdrawal symptoms and therefore be less reinforcing. However, a converse argument, based on the viewpoint of addiction outlined above, would be the following—if the driving need for hedonic homeostasis persists, then there would be an increased risk that as nicotine fails to serve the need, other drugs might be sought after to fill the need. The danger that one would merely be swapping drugs needs to be appreciated.
Altering nicotine metabolism
Once it enters the bloodstream, nicotine is rapidly metabolized in the liver. The immediate reaction step is the conversion of nicotine to its Δ1′(5′)—iminium form. This conversion is catalyzed by the Cytochrome P450 enzyme CYP2A6. The iminium form is rapidly oxidized to a major nicotine metabolite cotinine. CYP2A6 is the key enzyme in this pathway, also catalyzing the conversion of cotinine to t-3′-Hydroxycotinine (Hukkanen et al. 2005; Dempsey et al. 2004). CYP2A6 is a highly polymorphic gene, with at least 23 numbered variants differing in their expression, stability and activity. In a manner analogous to Alcohol dehydrogenase, these variants determine the clearance rate of nicotine and thus affect smoking behaviors. (Nakajima and Yokoi, 2005; Yamanaka et al. 2005). In a few studies methoxsalen, a CYP2A6 inhibitor reduced subjects’ desire to smoke thus increasing latency to the lighting of the next cigarette (Siu and Tyndale, 2008; Sellers et al. 2003a; Sellers et al. 2003b).
Further studies are under way evaluating the efficacy of CYP inhibitors coupled with other approaches like nicotine replacement therapy.
nAChR agonists and antagonists
The use of nAChR agonists and antagonists has been the area targeted by the bulk of pharmaceutical research over the last few years. A number of candidate drugs have been developed that are either in the market or at various testing stages.
Varenicline, a drug based on the alkaloid cytisine, is a partial agonist at the α4β2*-nAChRs and a full agonist at α7-nAChRs (Mihalak et al. 2006) and has been approved by the FDA for smoking cessation treatments. While the relative action of this drug on the two nAChR subtypes vis a vis its efficacy in smoking cessation is not clear, it is assumed that it alters the mesolimbic reward system by modulating dopamine release (Stack, 2007).
The marine worm toxin, anabasine has been the base compound for other drugs aimed at targeting nAChR effects. Two benzylidine derivatives of this compound GTS-21 and 4OH GTS-21, which have specific agonistic properties for the α7-nAChRs (Uteshev et al. 2003), are under Phase II trials for the treatment of Schizophrenia (Freedman et al. 2008). An anti-emetic drug, Tropisetron, has also shown potential for the treatment of auditory gating deficits in Schizophrenia (Koike et al. 2005).
Other approaches
There are other treatment options being considered to treat nicotine addiction that use other, potentially downstream targets of the drug (see George and O’Malley, 2004). These demonstrate significant efficacy, either by themselves or in combination with other treatments. The hedonic homeostasis view of drug abuse postulates that addiction is a form of mood disorder. Consistent with this viewpoint, a number of drugs like antidepressants have been found to be efficacious in combating nicotine addiction.
Smokers exhibit lower levels of MAO levels compared to non-smokers (Fowler et al. 1996; Fowler et al. 2003). As MAOs are key enzymes in modulating dopamine levels in the brain, it was postulated that maintaining low levels of the enzyme would be useful in combating nicotine addiction. The MAO inhibitor Moclobemide has been shown to help smoking cessation (Berlin et al. 1995b; Berlin et al. 1995a). Another MAO inhibitor Selegiline, has shown efficacy in smoking cessation (George et al. 2003), though a recent study shows the drug to also be a potent inactivator of CYP2A6 in humans (Siu and Tyndale, 2008).
Tricyclic antidepressants, like Nortriptyline, have been shown to reduce craving symptoms during early smoking abstinence periods (Prochazka et al. 1998; Hall et al. 1998). Similarly, the more atypical monoamine transporter blocker and antidepressant, Bupropion, has been marketed as aid for smoking cessation (Hays et al. 2001; Hurt et al. 1997). The μ-opiod receptor antagonist, naltrexone, has been shown to reduce nicotine craving when provided in conjunction with transdermal nicotine patches (Wong et al. 1999), by acting as an anxiolytic and antidepressant (Jarvekulg and Viru, 2002) and, possibly, by acting as an open channel blocker of the α7-nAChRs (Almeida et al. 2000).
Future attempts at using individually tailored combinations of these various approaches might lead to more effective therapeutic options to combat both smoking behaviors and diseases like AD and PD. This remains to be seen.
Conclusion
The importance of combating nicotine addiction is unquestionable. A number of nAChR agonists and antagonists are candidates for the treatment of addiction and neurodegenerative disorders. However, the widespread distribution of nAChRs adds a degree of caution in this regard. It should be recognized that understanding the physiological context in which these receptors work and consequences of functional disruption must give us pause. While it would be impractical to halt drug development until physiology is known, it is also imperative that we pay very close attention to the possibility of unintended consequences of such drugs.
Another important paradigm shift would be the recognition of addiction as a non-linear process with multiple possibilities of homeostatic disruptions leading to a similar phenotype. This view would imply that any single drug would be effective only in a small subpopulation of addicts and investing resources for a universal treatment for nicotine addiction might be a futile exercise.
Acknowledgments
Funding for this work was provided by grants from the National Institute of Drug Abuse. (RO1 DA 10266 and a CEBRA Grant 5 R21 DA019453) and the National Institute for Deafness and Communication Disorders (RO1DC008855) to S.V. and the American Heart Association Scientist Development grant to G.S.
Footnotes
Disclosure
The authors report no conflicts of interest.
Reference
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–61. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–73. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. III. Agonist actions of the novel alkaloid epibatidine and analysis of type II current. J Pharmacol Exp Ther. 1995;274:771–82. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–42. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LE, Pereira EF, Alkondon M, Fawcett WP, Randall WR, Albuquerque EX. The opioid antagonist naltrexone inhibits activity and alters expression of alpha7 and alpha4beta2 nicotinic receptors in hippocampal neurons: implications for smoking cessation programs. Neuropharmacology. 2000;39:2740–55. doi: 10.1016/s0028-3908(00)00157-x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha 7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–77. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–74. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–81. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Launay JM, Olivares R, Millet V, Lecrubier Y, Puech AJ. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clin Pharmacol Ther. 1995a;58:444–52. doi: 10.1016/0009-9236(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, Puech AJ. Monoamine oxidase A and B. activities in heavy smokers. Biol Psychiatry. 1995b;38:756–61. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Elmslie F, Hughes E, Trounce J, Sander T, Bertrand S, Steinlein OK. The CHRNB.2 mutation I312M is associated with epilepsy and distinct memory deficits. Neurobiol Dis. 2005;20:799–804. doi: 10.1016/j.nbd.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Weiland S, Berkovic SF, Steinlein OK, Bertrand D. Properties of neuronal nicotinic acetylcholine receptor mutants from humans suffering from autosomal dominant nocturnal frontal lobe epilepsy. Br J Pharmacol. 1998;125:751–60. doi: 10.1038/sj.bjp.0702154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford PC, Luntz-Leybman V, Freedman R. Auditory sensory gating in the rat hippocampus: modulation by brainstem activity. Brain Res. 1993;607:33–8. doi: 10.1016/0006-8993(93)91486-c. [DOI] [PubMed] [Google Scholar]
- Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson’s disease striatum. Mol Pharmacol. 2007;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, Sullivan B, Demasters BK, Freedman R, Leonard S. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–98. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, Bertrand D. Human alpha4beta2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: A patch-clamp study. J Neurosci. 1996;16:7880–91. doi: 10.1523/JNEUROSCI.16-24-07880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–17. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9:3097–101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–47. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann MF, Cerny T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court JA, Perry EK, Spurden D, Griffiths M, Kerwin JM, Morris CM, Johnson M, Oakley AE, Birdsall NJ, Clementi F. The role of the cholinergic system in the development of the human cerebellum. Brain Res Dev Brain Res. 1995;90:159–67. doi: 10.1016/0165-3806(96)83496-1. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res. 1993;10:131–41. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–24. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Lima PA, Wonnacott S. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology. 2000;39:2799–807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21:4125–33. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen ZM, Mansvelder HD, Keath JR, McGehee DS. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann NY Acad Sci. 2003;1003:185–95. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Dendritic Ca2+ signalling due to activation of alpha 7-containing nicotinic acetylcholine receptors in rat hippocampal neurons. J Physiol. 2007;582:597–611. doi: 10.1113/jphysiol.2007.135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–59. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B. in the brains of smokers. Nature. 1996;379:733–6. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998;18:8228–35. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–92. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial Phase 2 Trial of a Nicotinic Agonist in Schizophrenia. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Dunn D, Weiss R, Meyer EL, Rogers SW. Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J Comp Neurol. 2004a;468:334–46. doi: 10.1002/cne.10943. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Rogers SW. Neuronal and astrocyte expression of nicotinic receptor subunit beta4 in the adult mouse brain. J Comp Neurol. 2004b;468:322–33. doi: 10.1002/cne.10942. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Rogers SW. Mouse strain-specific changes in nicotinic receptor expression with age. Neurobiol Aging. 2005;26:973–80. doi: 10.1016/j.neurobiolaging.2004.07.005. [DOI] [PubMed] [Google Scholar]
- George TP, O’Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25:42–8. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Jatlow PI, Kosten TR, O’Malley SS. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biol Psychiatry. 2003;53:136–43. doi: 10.1016/s0006-3223(02)01454-3. [DOI] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2′-(14)C]nicotine. Drug Metab Dispos. 2001;29:645–51. [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–5. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rubio JM, Rojo J, Tapia L, Maneu V, Mulet J, Valor LM, Criado M, Sala F, Garcia AG, Gandia L. Activation and blockade by choline of bovine alpha7 and alpha-3beta4 nicotinic receptors expressed in oocytes. Eur J Pharmacol. 2006;535:53–60. doi: 10.1016/j.ejphar.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Buisson B, Touma E, Giordano T, Campbell JE, Hu IC, Donnelly-Roberts D, Arneric SP, Bertrand D, Sullivan JP. Stable expression and pharmacological properties of the human alpha 7 nicotinic acetylcholine receptor. Eur J Pharmacol. 1995;290:237–46. doi: 10.1016/0922-4106(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Gopalaswamy AK, Morgan R. Smoking in chronic schizophrenia. Br J Psychiatry. 1986;149:523. [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–46. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine [see comments] Nature. 1996;383:713–6. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–90. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA, Jr, Champtiaux N, Changeux JP. Localization of nAChR. subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci. 2000;12:3664–74. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, Sachs DP, Wolter TD, Buist AS, Johnston JA, White JD. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med. 2001;135:423–33. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol (Lond) 1999;515(Pt 3):769–76. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–9. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: implications for drug development. Neurosci Biobehav Rev. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Jarvekulg A, Viru A. Opioid receptor blockade eliminates mood effects of aerobic gymnastics. Int J Sports Med. 2002;23:155–7. doi: 10.1055/s-2002-23168. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Recent developments in the pathology of Parkinson’s disease. J Neural Transm Suppl. 2002:347–76. doi: 10.1007/978-3-7091-6139-5_33. [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–90. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–41. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–31. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Khiroug L, Yakel JL. Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes. Neuroscience. 2004;124:817–22. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci USA. 2006;103:19152–7. doi: 10.1073/pnas.0608215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Paired-pulse potentiation of alpha7-containing nAChRs in rat hippocampal CA1 stratum radiatum interneurones. J Physiol. 2005;568:881–9. doi: 10.1113/jphysiol.2005.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J Physiol. 2006;576:865–72. doi: 10.1113/jphysiol.2006.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove dA, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Hashimoto K, Takai N, Shimizu E, Komatsu N, Watanabe H, Nakazato M, Okamura N, Stevens KE, Freedman R, Iyo M. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res. 2005;76:67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–6. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–97. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–85. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R. Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–42. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, Pentel PR. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006a;184:409–16. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J. 2006b;8:E65–E75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson’s and Alzheimer’s diseases. Biochim Biophys Acta. 1999;1454:143–52. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang J, Zhu H, Qin C, Chen Q, Zhao B. Dissecting the signaling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer disease model. FASEB J. 2007;21:61–73. doi: 10.1096/fj.06-5841com. [DOI] [PubMed] [Google Scholar]
- Lobron C, Wevers A, Damgen K, Jeske A, Rontal D, Birtsch C, Heinemann S, Reinhardt S, Maelicke A, Schroder H. Cellular distribution in the rat telencephalon of mRNAs encoding for the alpha 3 and alpha 4 subunits of the nicotinic acetylcholine receptor. Brain Res Mol Brain Res. 1995;30:70–6. doi: 10.1016/0169-328x(94)00279-n. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol. 2003;480:117–23. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Marini C, Guerrini R. The role of the nicotinic acetylcholine receptors in sleep-related epilepsy. Biochem Pharmacol. 2007;74:1308–14. doi: 10.1016/j.bcp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Santos AD, Olivera BM. Conus peptides targeted to specific nicotinic acetylcholine receptor subtypes. Annu Rev Biochem. 1999;68:59–88. doi: 10.1146/annurev.biochem.68.1.59. [DOI] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1120–33. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–96. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci USA. 2004;101:9822–6. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Merlo PE, Chiamulera C, Bunnemann B. Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur J Neurosci. 2002;16:1633–46. doi: 10.1046/j.1460-9568.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yokoi T. Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab Pharmacokinet. 2005;20:227–35. doi: 10.2133/dmpk.20.227. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–50. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of drug addiction. Neuropsychopharmacology. 1994a;11:77–87. doi: 10.1038/npp.1994.37. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of drug addiction. Neuropsychopharmacology. 1994b;11:77–87. doi: 10.1038/npp.1994.37. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Nicke A, Wonnacott S, Lewis RJ. Alpha-conotoxins as tools for the elucidation of structure and function of neuronal nicotinic acetylcholine receptor subtypes. Eur J Biochem. 2004;271:2305–19. doi: 10.1111/j.1432-1033.2004.04145.x. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, Leslie FM, LaFerla FM. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2005;102:3046–51. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J Physiol Paris. 2006;99:172–9. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- OLDS J. Effects of hunger and male sex hormone on self-stimulation of the brain. J Comp Physiol Psychol. 1958a;51:320–4. doi: 10.1037/h0040783. [DOI] [PubMed] [Google Scholar]
- OLDS J. Satiation effects in self-stimulation of the brain. J Comp Physiol Psychol. 1958b;51:675–8. doi: 10.1037/h0039616. [DOI] [PubMed] [Google Scholar]
- OLDS J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958c;127:315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- OLDS J, OLDS ME. Positive reinforcement produced by stimulating hypothalamus with iproniazid and other compounds. Science. 1958;127:1175–6. doi: 10.1126/science.127.3307.1175. [DOI] [PubMed] [Google Scholar]
- Ostermann CH, Grunwald J, Wevers A, Lorke DE, Reinhardt S, Maelicke A, Schroder H. Cellular expression of alpha 4 subunit mRNA of the nicotinic acetylcholine receptor in the developing rat telencephalon. Neurosci Lett. 1995;192:21–4. doi: 10.1016/0304-3940(95)11598-q. [DOI] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D. Neuronal nicotinic alpha 7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol (Lond) 1996;491(Pt 1):151–61. doi: 10.1113/jphysiol.1996.sp021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, Stokes C. Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology. 2008;54:1189–200. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Phillips HA, Favre I, Kirkpatrick M, Zuberi SM, Goudie D, Heron SE, Scheffer IE, Sutherland GR, Berkovic SF, Bertrand D, Mulley JC. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet. 2001;68:225–31. doi: 10.1086/316946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HA, Marini C, Scheffer IE, Sutherland GR, Mulley JC, Berkovic SF. A de novo mutation in sporadic nocturnal frontal lobe epilepsy. Ann Neurol. 2000;48:264–7. [PubMed] [Google Scholar]
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med. 1998;158:2035–9. doi: 10.1001/archinte.158.18.2035. [DOI] [PubMed] [Google Scholar]
- Pugh PC, Margiotta JF. Nicotinic acetylcholine receptor agonists promote survival and reduce apoptosis of chick ciliary ganglion neurons. Mol Cell Neurosci. 2000;15:113–22. doi: 10.1006/mcne.1999.0810. [DOI] [PubMed] [Google Scholar]
- Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther. 1998;287:1136–44. [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F. Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci. 1994;6:1596–604. doi: 10.1111/j.1460-9568.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF. The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine Tob Res. 2003a;5:891–9. doi: 10.1080/14622200310001615231. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Tyndale RF, Fernandes LC. Decreasing smoking behaviour and risk through CYP2A6 inhibition. Drug Discov Today. 2003b;8:487–93. doi: 10.1016/s1359-6446(03)02704-1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Sharma G, Grybko M, Vijayaraghavan S. Action Potential-Independent and Nicotinic Receptor-Mediated Concerted Release of Multiple Quanta at Hippocampal CA3-Mossy Fiber Synapses. J Neurosci. 2008;28:2563–75. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA. 2001;98:4148–53. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–39. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–43. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–96. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Siu EC, Tyndale RF. Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice. J Pharmacol Exp Ther. 2008;324:992–9. doi: 10.1124/jpet.107.133900. [DOI] [PubMed] [Google Scholar]
- Sorenson EM, Chiappinelli VA. Localization of 3H-nicotine, 125I-kappa-bungarotoxin, and 125I-alpha- bungarotoxin binding to nicotinic sites in the chicken forebrain and midbrain. J Comp Neurol. 1992;323:1–12. doi: 10.1002/cne.903230102. [DOI] [PubMed] [Google Scholar]
- Stack NM. Smoking cessation: an overview of treatment options with a focus on varenicline. Pharmacotherapy. 2007;27:1550–7. doi: 10.1592/phco.27.11.1550. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–83. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–10. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Grutter T, Prado dC, Karplus M, Changeux JP. Implications of the quaternary twist allosteric model for the physiology and pathology of nicotinic acetylcholine receptors. Proc Natl Acad Sci USA. 2006;103:16965–70. doi: 10.1073/pnas.0607477103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper Y, Whyte D, Cahir E, Lester HA, Grady SR, Marks MJ, Cohen BN, Fonck C, McClure-Begley T, McIntosh JM, Labarca C, Lawrence A, Chen F, Gantois I, Davies PJ, Petrou S, Murphy M, Waddington J, Horne MK, Berkovic SF, Drago J. Nicotine-induced dystonic arousal complex in a mouse line harboring a human autosomal-dominant nocturnal frontal lobe epilepsy mutation. J Neurosci. 2007;27:10128–42. doi: 10.1523/JNEUROSCI.3042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. J Neurophysiol. 2003;89:1797–806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–62. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta 1 –42 binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000;75:1155–61. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- Warburton DM. Nicotine as a cognitive enhancer. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:181–91. doi: 10.1016/0278-5846(92)90069-q. [DOI] [PubMed] [Google Scholar]
- Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Lett. 1990;270:45–8. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD. A randomized trial of naltrexone for smoking cessation. Addiction. 1999;94:1227–37. doi: 10.1046/j.1360-0443.1999.948122713.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Nakajima M, Fukami T, Sakai H, Nakamura A, Katoh M, Takamiya M, Aoki Y, Yokoi T. CYP2A6 AND CYP2B.6 are involved in nornicotine formation from nicotine in humans: interindividual differences in these contributions. Drug Metab Dispos. 2005;33:1811–8. doi: 10.1124/dmd.105.006254. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind alpha-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–77. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]