TABLE 1.
Effect of Reaction Conditions on Condensation Reactions of 3 with 2-Naphthaldehyde
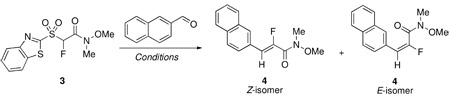 | |||||||
|---|---|---|---|---|---|---|---|
| entry | base | solvent | aldehyde:sulfone: base (molar equiv) | additive (molar equiv) | T, rxn time (h) | (%) E/Z ratio;a yieldb | |
| 1 | DBU | CH2Cl2 | 1.0:1.2:4.0 | -- | rt, 2 | 33/67; 90% | |
| 2 | DBU | CH2Cl2 | 1.0:1.2:4.0 | -- | −78 °C, 3.5 | 50/50; 96% | |
| 3 | DBU | THF | 1.0:1.2:4.0 | -- | rt, 2 | 19/81; 84% | |
| 4 | DBU | THF | 1.0:1.2:4.0 | -- | −78 °C, 4 | 4/96; 84% | |
| 5 | DBU | PhMe | 1.0:1.2:4.0 | -- | rt, 48 | 16/84; NAc | |
| 6 | DBU | DMF | 1.0:1.3:2.6 | -- | rt, 20 | 74/26; NAc | |
| 7 | DBU | DMF | 1.3:1.0:2.0 | -- | rt, 15 | 74/26; NAc | |
| 8 | DBU | DMPU | 1.3:1.0:2.0 | -- | rt, 17 | 78/22; 93% | |
| 9 | DBU | DMF-DMPUd | 1.3:1.0:2.0 | -- | −78 °C, 3.5 | 25/75; NAc | |
| 10 | DBU | DMPU | 1.3:1.0:2.0 | -- | 75 °C, 2.5 | 78/22; NAc | |
| 11 | DBU | THF | 1.0:1.3:3.9 | MgBr2 (1.8) | rt, 48 | 10/90; 78% | |
| 12 | DBU | THF | 1.2:1.0:3.0 | MgBr2 (1.4) | rt, 48 | 7/93; 78% | |
| 13 | DBU | THF | 1.2:1.0:3.0 | ZnBr2 (1.4) | rt, 48 | 15/85; 74% | |
| 14 | NaH | THF | 1.0:1.3:2.6 | -- | rt, 20; 70 °C, 2 | Only Z, 84% | |
| 15 | NaH | THF | 1.0:2.0:4.0 | -- | rt, 1 | Only Z, 90% | |
| 16 | NaH | THF | 1.0:2.0:4.0 | 18-Crown-6 (6.0) | rt, 1.5 | 40/60, NAc | |
| 17 | NaH | THF | 1.0:2.0:4.0 | 15-Crown-5 (6.0) | rt, 1.5 | 12/88, NAc | |
| 18e | K2CO3 | DMF | 1.0: 2.0:18.0 | TBAB (0.2) | rt, 18 | 55/45, NAc | |
Relative ratio of diastereomers in the crude reaction mixture determined by 19F NMR prior to isolation. No change in ratio was observed after purification.
Yields of isolated, purified products.
Product not isolated from reaction mixture.
A 1:1 mixture of DMF and DMPU was used to prevent freezing at −78 °C.
Reaction conditions were identical to those described in ref. 11.
