TABLE 2.
DBU-Mediated Condensation Reactions of 3
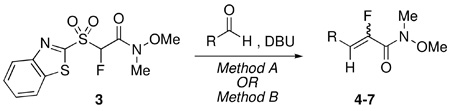 | ||||
|---|---|---|---|---|
| entry | RCHO | conditions, rxn time (h) | products 4–7: (%)E/Z ratio,a yieldb | δ (ppm); mult, J (Hz) |
| 1 | Method A, 4 | 4: 6/94;c 86% c | E-isomer:−110. 5; d, 21.3 | |
| 2 | Method B, 17 | 4: 78/22; 93% | Z-isomer: −120.5; d, 36.6 | |
| 3 | 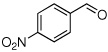 |
Method A, 2.5 | 5: 3/97; 92% | E-isomer:−104. 8; d, 21.3 |
| 4 | Method B, 16 | 5: 67/33; 83% | Z-isomer: −115.0; d, 33.6 | |
| 5 | Method A, 4 | 6: 74/26; 78% | E-isomer: −115.7; broad s | |
| 6 | Method B, 17 | 6: 86/14; 81% | Z-isomer: −119.7; d, 33.6 | |
| 7 | Method A, 2.5 | 7: 54/46; 74% | E-isomer: −117. 5; broad s | |
| 8 | Method B, 16 | 7: 67/33; 69% | Z-isomer: −125.7; d, 36.6 | |
Relative ratio of diastereomers in the crude reaction mixture determined by 19F NMR prior to isolation. No change in olefin ratio was observed after purification.
Yields of isolated, purified products (reactions were performed under similar conditions using either Method A or Method B, but were not optimized for individual cases).
Practically no change in E/Z ratio and yield was observed upon increasing the sulfone from 1.2 to 1.4 molar equiv (compare entry 4, Table 1).
