TABLE 3.
Condensation Reactions of 3 with Carbonyl Compounds Using NaH
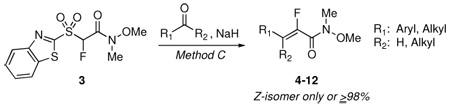 | ||||
|---|---|---|---|---|
| entry | R1CHO or R1C(O)R2 | conditions, rxn time | products 4–12: (%) E/Z ratio,a yieldb | Z-isomer: δ (ppm); mult, J (Hz) |
| 1 | Method C, 1 h | 4: Z-isomer only, 90% | −120.5; d, 36.6 | |
| 2 | Method C, 1.5 h | 8: 1:99%, 89% | −124.0; d, 36.6 | |
| 3 | 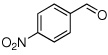 |
Method C, 1.5 h | 5: Z-isomer> 99; 85% | −115.0; d, 33.6 |
| 4 | 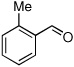 |
Method C, 1.5 h | 9: Z-isomer> 99%; 80% | −122.0; d, 36.6 |
| 5 | 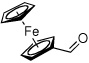 |
Method C, 1.5 h | 10: Z-isomer only; quantitative | −125.1; d, 36.6 |
| 6 | Method C, 1.5 h | 6: 2:98; 99% | −119.7; d, 33.6 | |
| 7 | Method C, 1.5 h | 7: Z-isomer> 99%; 83% | −125.7; d, 36.6 | |
| 8 | Method C, 1.5 h | 11: Z-isomer only; 71% | −124.7; d, 36.6 | |
| 9 | 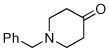 |
Method C,c 45 min | 12: NA; 57% | −126.3 (s) |
| 10 | 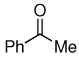 |
Method C, rt–heating, 8 h | Complex rxn mixture -products not identified | -- |
Relative ratio of diastereomers in the crude reaction mixture determined by 19F NMR prior to isolation.
Yields of isolated, purified products.
The ratio of N-benzylpiperidone:3:NaH = 2.5:1:2.
