TABLE 4.
Condensation Reactions of Fluoro Julia Reagents 16 and 17
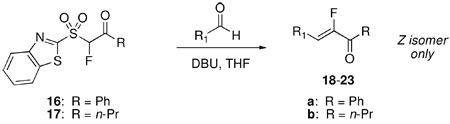 | ||||
|---|---|---|---|---|
| rxn | substrate | sulfone: molar equiv;a DBU: molar equiva | rxn time, T | Products 18–23, yieldb |
| 1 | 16: 3; DBU: 4 | 0.5 h, reflux | R = Ph: 18a, 61% | |
| 2 | 17: 3 ; DBU: 6 | 16 h, 0–5 °C | R = n-Pr. 18b, 89% | |
| 3 | 16: 4; DBU: 6 | 0.5 h, reflux | R = Ph: 19a, 64% | |
| 4 | 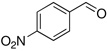 |
16: 2; DBU: 4 | 0.5 h, reflux | R = Ph:20a, 81% |
| 5 | 17: 2; DBU: 6 | 8 h, 0 °C - rt | R = n-Pr: 20b, 86% | |
| 6 | 16: 4; DBU: 3 | 40 min, reflux | R = Ph:21a, 71% | |
| 7 | 17: 3; DBU: 6 | 8 h, 0–5 °C | R = n-Pr: 22b, 90% | |
| 8 | 17: 1.5; DBU: 6 | 3 h, 0–5 °C | R = n-Pr: 23b, 73% | |
Total amount of sulfone and DBU used for complete aldehyde consumption. For good conversions, sequential addition of sulfone and DBU was required (please see the Experimental Section and the Supporting Information for details).
Yields of isolated, purified products.
