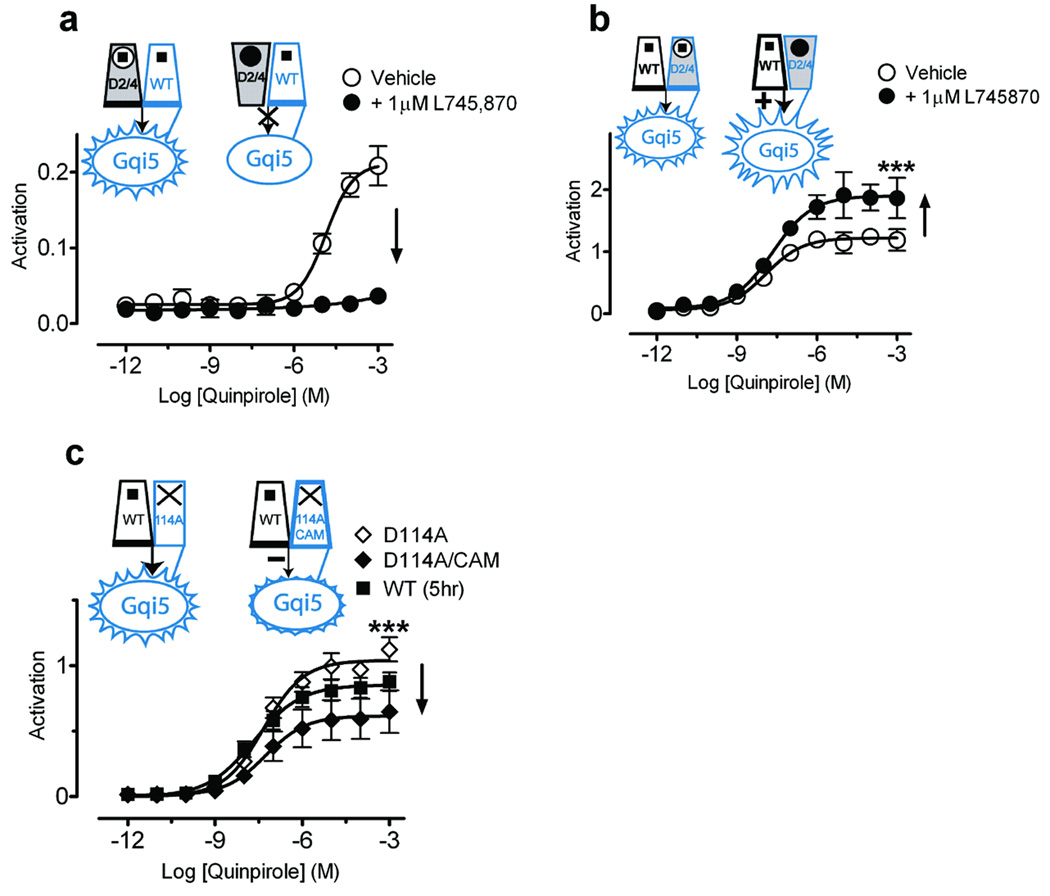
Figure 4. The second protomer allosterically modulates signaling.
Shown are effects on signaling with the D2/D4 construct expressed either as protomer A (a), or as protomer B (D2/D4-Gqi5) (b). (a) The D4-selective antagonist L745,870 (1 µM) totally blocked signaling of the D2/D4 construct expressed as protomer A with WT-Gqi5. (b) In contrast, L745,870 increased maximal activation for WT D2R coexpressed with D2/D4-Gqi5 to156.7±7.3% (n=9) (p<0.01*** by Student’s t-test) of that observed for D2R coexpressed with WT D2R-Gqi5 (Fig. 3a). (c) Coexpression of a constitutively active mutant (Supplementary Fig. 8 online) that was unable to bind ligand (D1143.32A/CAM-Gqi5), to enhance the fraction of protomer B in an active conformation, led to 49.6±8.4% (n=9) (p<0.01*** by Student’s t-test) of maximal activity (♦) when compared to WT D2R coexpressed with D114A-Gqi5 (◊). Activation data were normalized to surface expression as described in Fig. 3. The mean±SEM of at least 3 experiments, each conducted in triplicate, are shown.