Abstract
A class of thermosensitive biodegradable multiblock copolymers with acid-labile acetal linkages were synthesized from Pluronic® triblock copolymers (Pluronic® P85 and P104) and di-(ethylene glycol) divinyl ether. The novel polymers were engineered to form thermogels at body temperature and degrade in acidic environment. The Pluronic®-based acid-labile polymers were characterized using nuclear magnetic resonance, gel permeation chromatography and differential scanning calorimetry. In vitro biocompatibility of the synthesized polymers was evaluated using MTT assay. The polymers showed reverse thermogelling behavior in water around body temperature. The sol-gel transition temperatures of the polymers synthesized from Pluronic® P85 and P104 were lowered from 70.3 to 30oC and from 68.5 to 26.9oC, respectively, when the synthesized polymers were compared with corresponding Pluronic® block copolymers at a concentration of 25 wt%. The hydrophobic dye solubilization confirmed the formation of polymeric micelles in the aqueous solution. The sizes of multiblock copolymer increased upon a temperature rise indicating that thermal gelation was mediated by micellar aggregation. The thermally driven hydrogels showed preferential polymer degradation at acidic pH. At pH 5.0 and 6.5, the release of 40 kDa fluorescein isothiocyanate—dextran (FITC-dextran) from the thermally formed hydrogels was completed within 2 and 9 days, respectively. However, FITC-dextran was continuously released up to 30 days at neutral pH. The mechanism of FITC-dextran release at pH 5.0 was mainly an acid-catalyzed degradation whereas both diffusion and pH-dependent degradation resulted in FITC-dextran release at pH 6.5. The novel polymers hold great potential as a pH-sensitive controlled drug delivery system due to their interesting phase transition behavior and biocompatibility.
Keywords: thermosensitive, biodegradable, acid-labile, hydrogel, injectable
1. INTRODUCTION
Recent advances in smart polymeric materials have brought a great promise for the development of bioresponsive drug delivery systems which are able to release therapeutic molecules in response to the signals stemming from diseased tissues [1, 2]. Especially, biodegradable polymers which respond to temperature have been extensively considered as an effective injectable biomaterial for the delivery of small molecule drugs, proteins and genes [3-8]. These polymers are uniquely suited to minimally invasive therapeutic interventions by providing free flowing liquids below body temperature which turn into in situ gels upon injection [9, 10].
Block copolymers comprised of balanced hydrophilic and hydrophobic segments are known to undergo the thermosensitive phase transition in water [11]. Thermogelling biodegradable polymers based on block copolymers include poly(ethylene glycol)- poly(L-lactic acid) (PEG-PLLA) [3], poly(DL-lactic acid-co-glycolic acid)-poly(ethylene glycol)- poly(DL-lactic acid-co-glycolic acid) (PLGA—PEG—PLGA) copolymers (ReGel®) [10], PEG-PLGA-PEG [12], poly((DL-lactic acid-co-glycolic acid)-graft-(ethylene glycol)) (PLGA-g-PEG) copolymers [9], PEG-g-PLGA [13] and poly(ethylene glycol)-poly(caprolactone)-poly(ethylene glycol) (PEG-PCL-PEG) [14]. A simple aliphatic modification of biodegradable block copolymers has been a useful strategy to obtain thermosensitive polymers [15]. Poloxamer®, a non-biodegradable triblock copolymer of PEG-PPG-PEG, has also been used for the synthesis of slow eroding thermosensitive multiblock copolymer [11]. The thermosensitive Pluronic® block copolymers were also evaluated as drug delivery carriers [16].Three thermogelling multiblock copolymers, poly (ether-carbonate), poly (ether-ester), poly (ether-ester-carbonate), have been prepared by alternative/random connection of poly (ethylene glycol) and poly (propylene glycol) through carbonyl chloride and diacyl chloride coupling moieties [17]. These multiblock copolymers showed enhanced rheological properties after thermally driven gelation. The thermogelling polymers have consistently achieved controlled drug delivery via in situ depot formation [10]. Not only the release of small molecule drugs but also the release of macromolecules such as proteins, chondrocytes, plasmid DNA has been controlled with the thermogelling polymers [8, 10, 18].
More recently, dual-stimuli-sensitive polymers with thermosensitivity have been evolved [1,19-21]. Poly(2-diethylaminoethyl-methyl methacrylate)-poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide)-poly(2-diethylaminoethyl-methyl methacrylate), a pentablock copolymer which is pH-sensitive thermogelling polymer has been synthesized from Pluronic® F127 triblock copolymers via controlled atom transfer radical polymerization [19]. The pentablock copolymer exhibits pH-sensitive micellization and thermoreversible sol-gel transitions in water. Poly(β-amino ester) has also been used as a duo-functional group in the synthesis of poly(β-amino ester)-poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone)-poly(β-amino ester), a thermosensitive pH-sensitive pentablock copolymer [20]. The pentablock copolymer was synthesized by the coupling of pH-sensitive poly(β-amino ester) to thermosensitive biodegradable poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone). An interesting dual-stimuli-sensitive polymer has been based on acid-labile acetal linkages which have provided an effective mechanism of polymer biodegradation in an acidic medium [22]. Polyacetal-based temperature/pH-sensitive polymers were synthesized by grafting PEG to a polyacetal backbone [21]. The PEG-grafted polyacetal has formed a thermogel which favorably erodes at an acidic pH. Another thermosensitive polymer with dual sensitivity has been introduced by the incorporation of bioreducible disulfide bonds in the polymer backbone [1]. These dual sensitive polymers are advantageous over other thermosensitive polymers in that the release of a pharmacologically active agent can be triggered by an acidic milieu of a diseased tissue or intracellular compartments. The specific pH for the degradation of acetal-based polymers can be found in extracellular tumor fluid (pH 6.5 - 7.2) and endosomes (pH 5 – 6.5) which is lower than the pH of the normal tissues. [23-27].
Even though a variety of dual-stimuli-sensitive polymers have been reported, their syntheses usually require complicated multi-step reactions [19, 20]. In addition, the responses of the polymers to each stimulus are usually slow because of the chemico-structural restrictions for the dual sensitivity [21, 22]. The purpose of this study is to synthesize novel Pluronic®-based multiblock copolymers connected via acid-labile acetal bonds rendering facilitated pH-sensitive biodegradation. The hypothesis behind this novel polymer is that the combination of thermosensitive Pluronic® and acid-labile acetal linkages would result in a dual-sensitive polymer which can form gel at body temperature and allow bioresponsive polymer degradation at an acidic pH. In this body of work, we demonstrated the acid-catalyzed degradation of the polymers and the pH-dependent release of model compound, fluorescein isothiocyanate—dextran from thermally formed gel.
2. EXPERIMENTAL
2.1. Materials
Pluronic® P 85 and P104 were kindly provided from BASF Chemical Company (Florham Park, NJ). PTX was purchased from LC laboratories® (Woburn, MA). Polystyrene standards of molecular weights 1, 4, 20, 50 and 100K were obtained from Polysciences, Inc. (Warrington, PA). p-Toluenesulfonic anhydride, anhydrous tetrahydrofuran (THF), 1, 6-diphenyl-1, 3, 5-hexatriene (DPH) and anhydrous toluene were purchased from Acros Organics (Morris Plains, NJ). Di-(ethylene glycol) divinyl ether (DEGDVE) was obtained from Aldrich (Milwaukee, WI). FITC-Dextran of molecular weight 40kDa was purchased from Sigma (Saint Louis, MO). Petroleum ether and methylene chloride from Fisher (Pittsburgh, PA) were of analytical grade and used as supplied.
2.2. Polymer synthesis
Fifteen grams of Pluronic® P85 (MW 4600) or P104 (MW 5900), and 0.03 molar equivalent of p-toluene sulfonic anhydride to Pluronic® were dissolved in 150 mL of anhydrous toluene. The mixture was dried by distilling off 100 mL of toluene. DEGDVE was added to the dry mixture at a 1:1 molar ratio to Pluronic®. The reaction was carried out overnight by magnetic stirring at 50oC under dry nitrogen. Upon completion of the reaction, the reaction solution was mixed with 150 mL of petroleum ether to precipitate polymer. The polymer precipitate was dissolved in a dilute sodium hydroxide solution at a 2:1 molar ratio of NaOH to p-TSA to achieve a concentration of 15 wt% at 4oC. The aqueous polymer solution was heated to 75oC to precipitate the polymer. After dissolving the crude precipitated polymer in distilled water at a concentration of 20 wt%, the polymer was precipitated again by heating. The polymer was purified by another cycle of dissolving by cooling and precipitation by heating. The final precipitate was freeze-dried to obtain white powder. Two different thermosensitive Pluronic®-based multiblock copolymers (MBCPs), MBCP-1 from Pluronic® P85 and MBCP-2 from Pluronic® P104, were finally obtained. The yields of the reactions were 90% and 87%.
2.3. Polymer characterization
Chemical structures of the synthesized polymers were analyzed by 1H-NMR. Polymers were dissolved in CDCl3 and recorded on a 400MHz NMR spectrometer (Bruker UltrashieldTM 400 PLUS, Germany). Molecular weights of the synthesized MBCPs and Pluronic® copolymers were determined by gel permeation chromatography (GPC). GPC was run on a Waters system equipped with a binary pump (Waters 1525), a refractive index detector (Waters 2414), and a Styragel HR4E column (300 × 7.8 mm I.D., 5 μm particle size). THF as a mobile phase was eluted at a flow rate of 1.00 mL/min at 25oC. Polystyrene standards in the molecular weight range of 4,000-100,000 Daltons were also run to obtain a calibration curve. Molecular weights of the synthesized polymers were calculated from the obtained calibration curve. Melting temperatures of the synthesized polymers were determined by diffrential scanning calorimetry (DSC). Thermograms were obtained by running the polymer samples sealed in aluminum pans on a Diamond differential scanning calorimeter (Perkin Elmer, Waltham, MA) in the temperature range of -30 to 100oC with a heating and cooling rate of 5oC/min. Initially the samples were equillibriated at -30oC for 15 min. Dry nitrogen was continuously supplied at a flow rate of 20 mL/min during the analysis.
2.4. Critical micelle concentration (CMC) of MBCP
The CMCs of MBCPs were determined by a dye solubilization method with 1, 6-diphenyl-1, 3, 5-hexatriene (DPH) [11]. DPH of 0.4 mM in methanol was used in this CMC determination. Stock solutions of MBCP-1 and -2 were prepared at a concentration of 10 wt%. Polymer solutions in the concentration range of 10.0 to 1.0 × 10-5 wt% were prepared by serial dilutions of the stock solutions. Then, 25 μL of the DPH solution was added to 2.5 mL of each polymer solution. The polymer solutions were incubated for 24 h in a dark place. The absorbencies of the test solutions were recorded on a Lambda EZ201 UV spectrophotometer (Perkin Elmer, Waltham, MA) at wavelengths 377 and 391nm.
2.5. Degradation of MBCP
The MBCP solutions were prepared at a concentration of 25 wt% in deionized water. The polymer solution (100μL) was placed in 1mL vials and the vials were kept at 4oC overnight. Later, the polymer solutions were incubated at 37oC for 15 min for gelation. After gelation, 200μL of phosphate buffer (pH 5.0, 6.5 and 7.4) was added to the vials. After gel degradation for a predetermined time, the gels were frozen and lyophilized for GPC measurements. The dried polymer was dissolved in 1ml dichloromethane and centrifuged at 10000 rpm for 10 min to remove the buffer components. The supernatants were collected and vacuum dried to prepare GPC samples. GPC was run to determine the distribution of polymer molecular weight.
2.6. In vitro cytotoxicity of MBCP
Calorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to test the cytotoxicity of MBCP-1 and MBCP-2 on NIH 3T3 mouse embryonic fibroblast cells which have been widely used for the evaluation of in vitro biocompatibility of biomaterials [28-30]. Initially, NIH 3T3 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) and antibiotics. The cultured cells were seeded in 96 well plates at a density of 5 × 103 cells/well in 0.2 ml of the cell culture medium and grown for 24 h at 37oC. After removing the culture medium, 100 μl of an MBCP solution of which concentration ranged from 0.1 to 5.0 % (w/v) in DMEM supplemented with 10 % FBS were placed on the top of the cell layer. The cells were incubated for an additional 48 h and the metabolic activity of the cells was measured. After removal of the test solution, 100 μl of fresh culture medium containing 50 μg MTT was added to each well and the cells were incubated for 4 h. Lysis buffer comprising of 10 % (w/v) SDS and 45 % (v/v) DMF was then added and incubated for another 24 h. Absorbance was measured at 590 nm on a Model 550 microplate spectrophotometer (Bio-Rad, Laboratories Inc., Hercules, CA). Cell viability was calculated as compared to phosphate buffered saline (PBS)-treated cells (100 % survival).
2.7. Dynamic light scattering
The apparent particle sizes and particle size distributions of the MBCPs were measured using dynamic light scattering. A concentration (4% w/v) above CMC was chosen to study the micellar aggregation as a function of temperature. Zetasizer Nano S (Malvern Instruments, Malvern, UK) was used to measure the particle size of the samples. The instrument was operated at four different temperatures such as 10, 20, 30 and 40oC.
2.8. Thermal gelation of MBCP solutions
2.8-1. Test tube inverting method
A series of MBCP solutions at concentrations of 5, 10, 15, 20 and 25 wt% were prepared in deionized water. One milliliter of each polymer solution was transferred to each 4mL vial and the vials were kept at 4oC overnight. The vials were then placed in a water bath and equilibrated at 15oC for 15 min. The sol-gel and gel-sol phase transitions of the polymer solutions were tested by tube inversions in the temperature range of 15 to 80oC while heating the polymer solutions at a rate of 2oC per every 5 min [1, 12, 13, 15]. The temperature at which a polymer solution stopped flowing upon a tube inversion was recorded as gelation temperature. Phase transitions of the solutions of Pluronic® P85, Pluronic® P104, MBCP-1 and MBCP-2 were determined by the method.
2.8-2. Falling ball method
The solutions of MBCP-1 and MBCP-2 were prepared at a concentration of 25 wt% in deinonized water. One milliliter of each polymer solution was placed in a NMR tube with a diameter of 4.0 mm. The time (t) required for a steel ball (diameter (D) =1.97 mm, density (ρ s) = 7.97 g/mL) to fall a specified distance (d = 3 cm) was measured with a temperature increment of 2oC per step for each polymer concentration. The polymer solutions were equilibrated for 20 min at each temperature. A graph was plotted between temperature and the dynamic viscosity. The velocity of the falling ball (v) can be calculated from the formula d/t. The density of the polymer solution (ρf) was assumed to be 1.0 g/cm3. The specific gravities of the sphere (γs) and the polymer solution (γf) were calculated using the formula γ = ρg (acceleration due to gravity (g) = 980 cm/s2). Finally the dynamic viscosity (μ) was calculated using the following formula
2.9. FITC-Dextran release
FITC-Dextran (40kDa) was dissolved in the polymer solutions at a concentration of 10 mg/ml. The solutions were vigorously stirred at 4oC until they turned into clear solutions. One milliliter of each FITC-Dextran-loaded MBCP solution was placed in amber colored vial and allowed for gelation at 37oC. Two milliliters of each phosphate buffer solution (pH 5.0, 6.5 and 7.4) were added to each vial. While incubating at 37oC media was changed periodically. The perfect sink conditions were maintained through the release study. Release media collected at each time point was analyzed by UV-visible spectroscopy at 490nm.
3. RESULTS & DISCUSSION
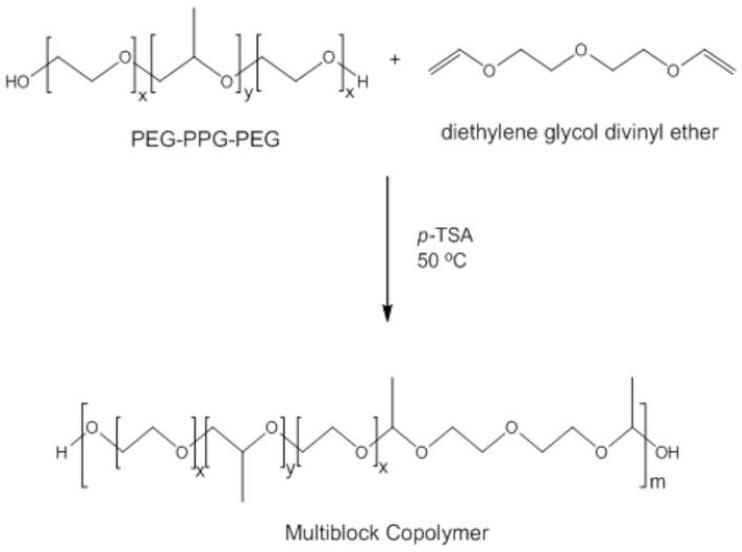
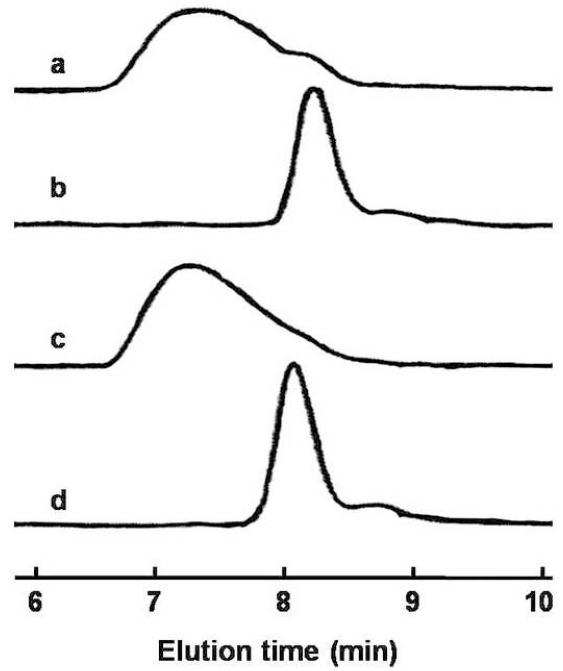
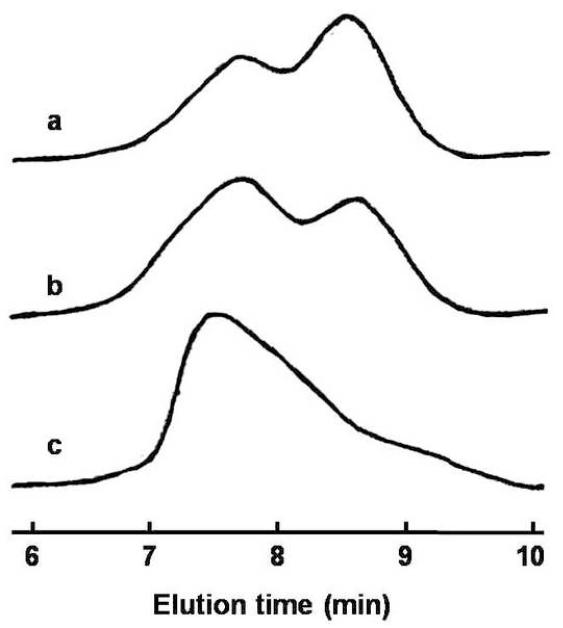
A facile approach to engineer a pH-sensitive thermogelling multiblock copolymer has been demonstrated in this research work. Pluronic® (P85 and P104), a group of triblock copolymer of PEG-PPG-PEG, has been used for the synthesis of acid-labile thermosensitive biodegradable MBCPs. Scheme 1 shows the synthetic reaction between Pluronic® copolymer and DEGDVE in presence of p-toluenesulfonic anhydride as a catalyst, which results in MBCP with acetal linkages. The reaction requires anhydrous conditions because a trace amount of water leads to a premature termination of the oligomerization, which results in low molecular weight MBCP [22]. Water molecules are known to compete with hydroxyls to form hemiacetal intermediates. Hence, the reactants were azeotropically dried to remove water adsorbed on Pluronic® prior to oligomerization. Polyacetal formation between divinyl ether and hydroxy-terminated monomers is favorable even at mild temperature [31-33]. At around 40°C, the polymerization is completed within 1 h. However, we have reacted Pluronic® and DEGDVE overnight at 50°C to ensure a successful oligomerization because Pluronic® of molecular weight around 5000 may not be readily accessible for the oligomerization. Thickening of the reaction mixture was observed in 4-5 h after the addition of DEGDVE. Upon completion of the oligomerization, p-TSA was immediately neutralized by dissolving the resulting polymer in a dilute sodium hydroxide solution in order to prevent polymer degradation by acid-catalyzed hydrolysis during purification. MBCPs were precipitated by heating the solution to 70°C. The polymers could be further purified in a complete aqueous environment by the cycle of cooling and heating, which would be advantageous for pharmaceutical applications of the polymers because harmful organic solvents can be avoided during the purification process. In addition, unreacted Pluronic® and DEGDVE could be removed by the polymer purification method. As shown in Figure 1, purified MBCPs include only trace amounts of the unreacted Pluronic® copolymers. Moreover, GPC chromatographs in Figure 2 shows that the purification process removed most of the remaining Pluronic®. Chromatograph of crude MBCP right after synthesis indicates that the crude polymer contains a significant amount of unreacted Pluronic® (Fig. 2a). However, the purification of the crude polymer via repetitive precipitation in hot water was able to remove most of free Pluronic® (Fig. 2c).
Scheme 1.
Schematic synthetic reactions for the preparation of MBCPs from PEG-PPG-PEG triblock copolymer (Pluronic®).
Figure 1.
Gel permeation chromatograms of (a) MBCP-1, (b) Pluronic® P85, (c) MBCP-2 and (d) Pluronic® P104. The retention times of the synthesized polymers (~8.1) were different from the Pluronic® copolymers (~9.2) indicating the formation of MBCPs.
Figure 2.
Gel permeation chromatograms of MBCP-2 (a) after the synthesis, (b) after the purification in aqueous NaOH and (c) after the purification in water.
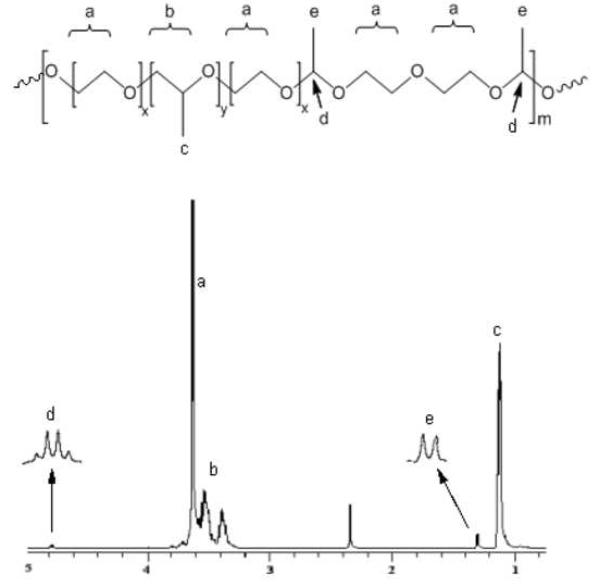
Chemical structures of the synthesized MBCPs were confirmed by NMR spectroscopy. The 1H-NMR spectra of Pluronic® P85 and P104 and their multiblock copolymers were obtained in CDCl3. A typical 1H-NMR spectrum of MBCP is shown in Figure 3. This NMR spectrum of MBCP-1 shows methyl protons of PPG (-CH2CH (CH3) O-) at 1.0-1.2 ppm, methylene and methine protons of PPG (-CH2CH (CH3) O-) at 3.2-3.6 ppm, and methylene protons of PEG (-CH2CH2O-) at 3.6 ppm. The acetal bond formation was confirmed by a quartet at 4.75 ppm assigned as methine protons of acetal groups and a doublet at 1.30 ppm from methyl protons of acetal groups. The characteristic NMR peaks of acetal groups are consistent with previously reported results [22]. In the case of divinyl linkers, hydrophobic linkers such as butanediol divinyl ether and cyclohexane dimethanol divinyl ether would replace hydrophilic DEGDVE. However, the incorporation of hydrophobic linkers between hydrophilic PEG blocks of Pluronic® would result in different gelation behavior from the MBCP synthesized with DEGDVE. The synthetic method demonstrated in this work is simple compared to previous syntheses for pH-sensitive thermogelling polymers. Previous synthesis of pH-sensitive thermogelling polymer involved multi-step reactions that included polyacetal synthesis and following PEG grafting [21]. Gel permeation chromatography was used to determine the number-average molecular weight (Mn) and polydispersity index of Pluronic® copolymers and MBCPs. These values are summarized in Table 1. The calculated molecular weights of MBCP-1 and MBCP-2 were 36920 (±1650) and 40210 (±860) g/mol, respectively. The GPC measurements also indicate that the synthesized polymers are multiblock copolymers with 4.9 and 4.1 Pluronic® units in MBCP-1 and MBCP-2 respectively. The reasons for the relatively low molecular weight oligomers may be the presence of trace water even after azeotropic distillation and limited availability of end hydroxyl groups of large Pluronic® molecules for the oligomerization. Based on DSC analysis, melting temperatures (Tm) of Pluronic® have been changed after the oligomerization in the presence of DEGDVE. Tm values of MBCP-1 and MBCP-2 were 29.0oC and 29.8oC respectively while Pluronic® P85 and P104 have Tm values of 38.9oC and 39.4oC respectively. The changes in Tm might be the result of interrupted polymer packing by the addition of acetal bonds between PEG blocks of Pluronic®.
Figure 3.
1H-NMR spectrum of MBCP-1. A quartet (d) at 4.75 ppm from acetal groups and a doublet (e) at 1.30 ppm from methyl protons of acetal groups indicated successful synthesis of MBCPs.
Table 1.
Characterization results of the synthesized MBCPs. The Number average molecular weights represent the mean ± SD for n=3.
| Polymer | Mn (g/mol)a | Mw/Mnb | Number of repeating units |
Tm (°C)c |
|---|---|---|---|---|
| Pluronic® P85 | 7440 (±130) (4600d) | 1.04 | - | 38.9 |
| Pluronic® P104 | 9880 (±170) (5900e) | 1.04 | - | 39.4 |
| MBCP 1 | 36920 (±1650) | 1.66 | 4.9 | 29.0 |
| MBCP 2 | 40210 (± 860) | 1.69 | 4.1 | 29.8 |
Number average molecular weights determined by GPC
Polydispersities based on GPC measurements
Melting temperatures determined by DSC
Molecular weights from BASF chemical company
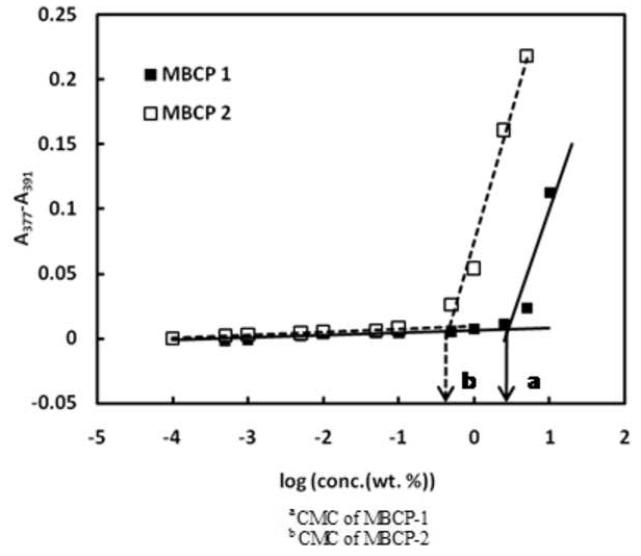
CMCs of MBCP-1 and MBCP-2 were determined by a hydrophobic dye solubilization method which has been frequently employed for the characterization of amphiphilic block copolymers [11]. DPH, a hydrophobic dye used in this study, has a tendency to partition into the hydrophobic core of the micelles in the presence of amphiphilic block copolymers. The solubility and UV absorbance of DPH sharply increase at the CMCs of the amphiphilic polymers. The CMCs of amphiphilic block copolymers can be calculated by extrapolating the absorbance versus logarithmic concentration (Figure 4). The CMCs of MBCP-1 and MBCP-2 were found to be 2.854 (±0.030) wt% and 0.485 (±0.009) wt%, respectively. The CMC difference between MBCP-1 and MBCP-2 is due to the difference in the ratio between hydrophobic PPG and hydrophilic PEG. MBCP-2 was expected to have a lower CMC than MBCP-1 because Pluronic® P104 has a greater PPG/PEG ratio than Pluronic® P85. In the case of amphiphilic block copolymers based on PEG and PPG, a block copolymer with a greater PPG/PEG ratio generally has a lower CMC [34].
Figure 4.
Determination of CMC of the synthesized MBCPs by the dye solubilization. The difference in the absorbance of DPH at 377 and 391nm was plotted on vertical axis. The cross-section point of the two exponential lines was defined as the CMC. The CMCs of MBCP-1 and MBCP-2 were 2.854 (± 0.030) wt% and 0.485 (± 0.009) wt%, respectively.
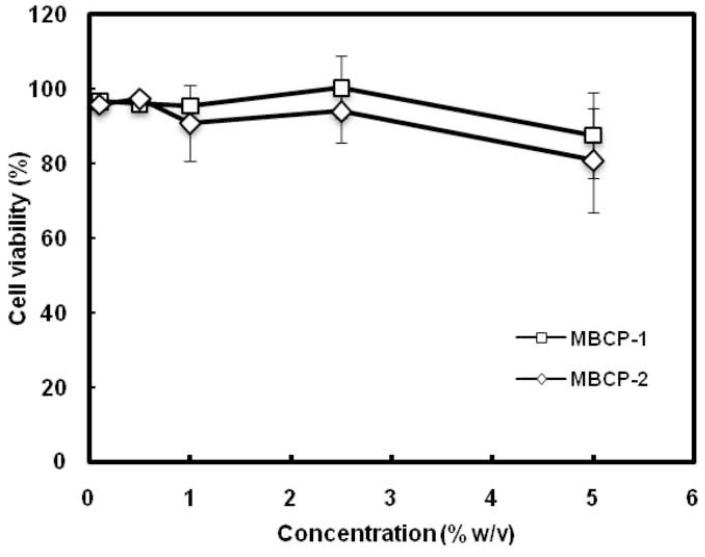
Cytotoxicity of the synthesized MBCPs has been evaluated by an MTT assay using NIH 3T3 mouse embryonic fibroblast cells. The MTT assay was performed in the concentration range 0.1 to 5.0% (w/v) and these results are shown in Figure 5. It was difficult to perform the assay above 2.5% (w/v) polymer concentration because of pronounced polymer aggregate formation at 37oC. During the experiment, the removal of polymer aggregates via pipetting resulted in a slight suction of testing cells, which might cause an underestimation of cell viability. Even with the presumed underestimation of cell viability, MBCP solutions showed overall cell viability greater than 80%. In addition, thermal gelation of MBCP is not expected to leave a significant amount of the polymer as unimers or micelles only which can penetrate cell membrane and thus affect cell viability. Taken together, the synthesized MBCPs are not considered as cytotoxic and, as such, hold great potential for biomedical and pharmaceutical applications. It is vital that polymer degradation products should also be non-toxic. Degradation of acetal bond yields an equal amount of acetaldehyde [22]. Due to very low amount of degraded products compared to the initial polymer, the cytotoxicity associated with the degraded products, is expected to be minimal.
Figure 5.
In vitro cytotoxicity of the synthesized MBCPs as a function of copolymer concentration. Cell viability was determined by an MTT assay using cultured NIH 3T3 mouse embryonic fibroblast cells. The results represent the means ± SD for n=3.
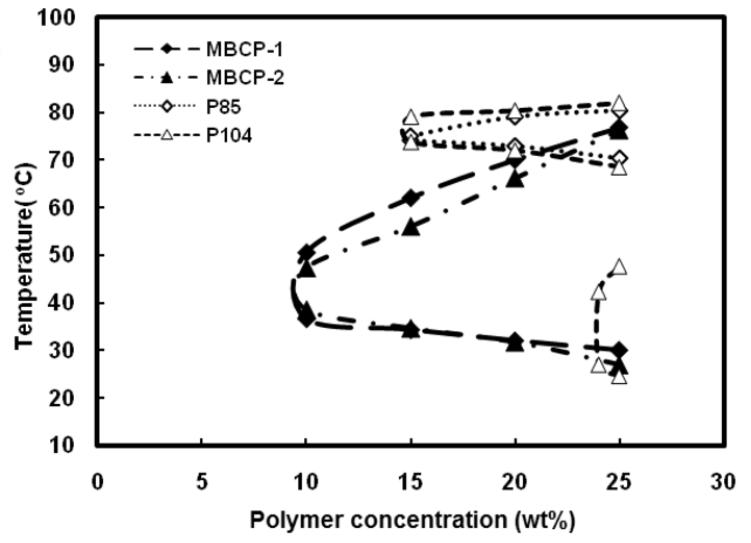
Phase transitions of aqueous solutions of Pluronic® copolymers and MBCPs were evaluated by a test tube inverting method. The sol-gel transitions of the polymeric solutions were determined by flow (sol) — no flow (gel) basis. Aqueous solutions of both MBCPs underwent sol-gel and gel-sol phase transitions upon temperature increments. As shown in Figure 6, the temperatures at which the phase transitions occurred were dependent on polymer concentration. Aqueous solutions of MBCPs were transparent at room temperature and turned into gels within two minutes as the temperature reached 30-37oC. These MBCP solutions in sol phases were free flowing liquids which could be injected through a 25-gauge needle. The immediate thermal gelation of the MBCP solutions around body temperature and their injectability via a small-bore needle make MBCPs particularly attractive for local drug delivery.
Figure 6.
Phase diagrams of the aqueous solutions of Pluronic® P85, Pluronic® P104, MBCP-1 and MBCP-2 determined by the test tube inverting method.
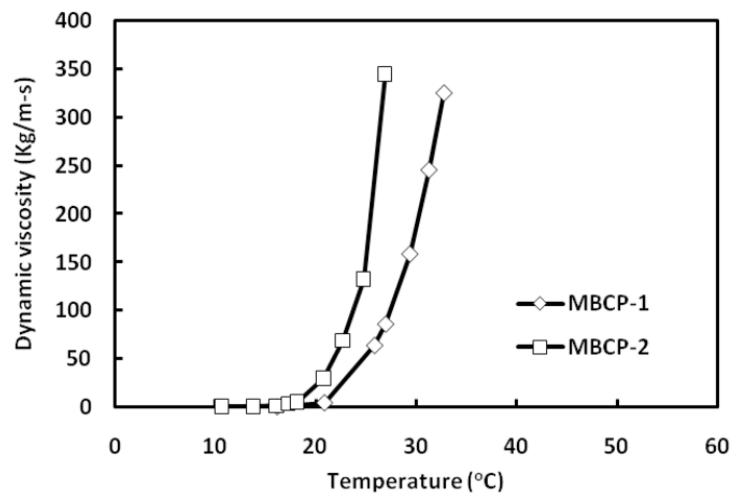
Even though several mechanisms have been proposed for the description of thermal gelation of block copolymer solutions, the mechanism based on ordered micelle packing has been most widely accepted [35-37]. Based on the ordered micelle packing mechanism, PPG blocks are dehydrated at a concentration above CMC and Pluronic® block copolymers form micelles. At this concentration, polymeric unimers and the micelles are in an equilibrium state. As the temperature increases, this equilibrium shifts from unimers to micelles, making the micelle volume fraction (Φm) considerably high. When Φm is greater than 0.53 in the case of Pluronic®, micelles come into contact and pack themselves into ordered structures leading to gelation [38]. Thermal gelation of MBCP solutions may be explained by the micelle packing. The solutions of Pluronic® P85, MBCP-1 and MBCP-2 showed sol-gel-sol phase transition in a concentration range up to 25 wt%. However, 25 wt% Pluronic® P104 solution possessed two distinct gel phases upon sol-gel-sol-gel transition. The first gel phase of Pluronic® P104 solution ranged from 24 to 47oC while the second phase ranged from 70 to 80oC. This may be due to the formation of different liquid crystalline phases, isotropic/cubic phases at low temperature and multi-phases (cubic/hexagonal/lamellar) at high temperature [39]. Gelation temperatures of MBCPs at which the polymer solution underwent sol-gel phase transition were different from those of their corresponding Pluronic®. Moreover, the critical gelation concentration (CGC) of MBCPs determined from Figure 6 was 10 wt%, which was lower than that of their Pluronic® copolymers, 15 wt%. The lowered CGC and altered gelation temperature after oligomerization is primarily due to extended and enforced chain entanglements in MBCPs compared to their corresponding Pluronic® copolymers [35, 40]. Based on the test tube inverting method, a polymer concentration of 25 wt% was chosen for the measurements of dynamic viscosity using falling ball method. Figure 7 shows the dynamic viscosity changes of MBCP-1 and MBCP-2 polymer solutions with temperature. Dynamic viscosity dramatically increased at 25 and 22oC for MBCP-1 and MBCP-2 respectively. In addition, the sol-gel transition temperatures determined by falling ball method were 25.02 and 22.36oC for MBCP-1 and MBCP-2 at 25 wt%. The transition temperatures determined by falling ball method were consistent with those values determined by test tube inverting method. A mere variation of 2 to 4 degrees was often observed between both the methods. The difference may be due to a longer time for equilibration for the falling ball method compared to the test tube inverting method. Even though the falling ball method would allow for more accurate and reliable transition temperature, the test tube inverting method would give better simulation of practical injection of a formulation with consideration of kinetic aspect of the process.
Figure 7.
Determination of dynamic viscosity changes of MBCP-1 and MBCP-2 aqueous solutions as a function of temperature using falling ball method. The polymer concentration was 25 wt%. The MBCP solutions were equilibrated for 20 minutes at each temperature.
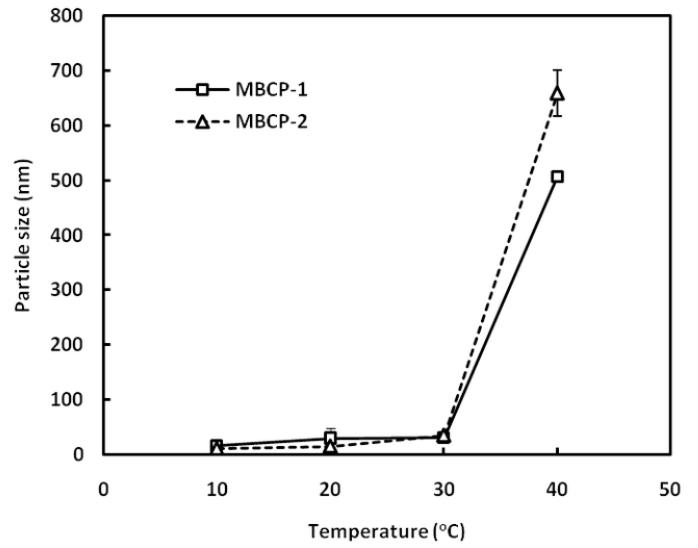
Figure 8 shows the micellar size change with temperature. Determined sizes of micelles or micellar aggregates ranged 16-30 nm and 11-35 nm in diameter for MBCP-1 and MBCP-2, respectively, at temperatures below 30oC. The micelle sizes were slightly increased as the temperature increased from 10 to 30oC. However, the micelle aggregation upon a temperature increase from 30 to 40oC resulted in a dramatic increase in micellar sizes to the range of 500-700 nm. This indicates that the micellar aggregation is the main mechanism for sol-gel transition of MBCP at 30-40oC.
Figure 8.
Determination of micellar size changes of MBCP-1 and MBCP-2 using dynamic light scattering. The micellar size changes were measured as a function of temperature. The polymer concentration was 4% w/v. The results represent the mean ± SD for n=3.
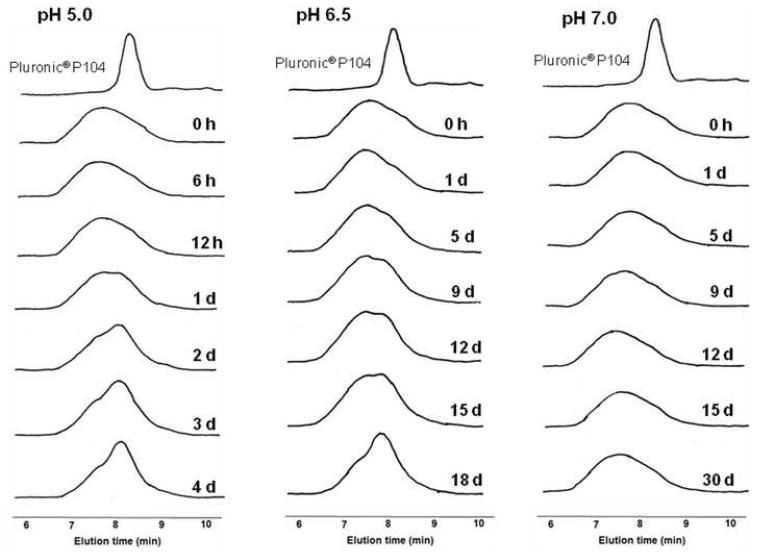
GPC chromatograms in figures 9 show the degradation of MBCP-2 at different pHs. Since the degradation pattern of both MBCP-1 and MBCP-2 was similar, only the degradation of MBCP-2 is shown here. The polymer degraded more rapidly in acidic media than in neutral medium. The polymers were not degraded at pH 7.4 for even 40 days while the complete degradation was observed within 4 and 18 days at pH 5.0 and 6.5, respectively. At pH 6.5, the polymer started degrading from day 5, which coincided with the initiation time of gel erosion. From these results, it is evident that the multiblock copolymers were degraded into lower molecular weight polymers. The degradation of hydrogel is generally affected by several factors such as the number of degradable bonds in the polymer [41, 42], the presence of cross-links [43] and characteristics of degradable bonds [21, 22]. The important factors for the degradation of the MBCP polymers are presumably the number of acid-labile acetal bonds and sensitivity of acetal bond to pH. Compared with previously reported PEG-grafted polyacetal-based polymers, MBCP polymers were degraded faster than the graft polymer-based polymers in an acidic medium. In the case of PEG-grafted polyacetal gel, it takes about 40 days for polymer degradation at pH 5.0. [21]. The main reason for the faster degradation may be the fewer acetal bonds in MBCPs than in the graft polymer. Because MBCPs have only 4 or 5 average acetal bonds in each polymer chain, the cleavage of small number of acetal bonds can result in Pluronic® unimers or low-molecular weight oligomers of which solutions have a greater CGC and/or higher gelation temperature than MBCPs. However, PEG-grafted polyacetal should be significantly degraded before losing thermosensitivity. Cleavage of small number of acetal bonds in polyacetal backbones would not considerably change the overall ratio of hydrophilicity to hydrophobicity in the PEG-grafted polyacetal. The possible degradation products of MBCPs would be initial triblock copolymer (PEG-PPG-PEG), and a little amount of diethylene glycol and acetaldehyde based on the inference from an acid-labile PEG polymer with acetal bonds [22]. Monomeric Pluronic® molecular weights are smaller than 60,000 which is the maximum molecular weight cleared by kidneys.
Figure 9.
Gel permeation chromatograms showing MBCP-2 polymer degradation at three different pH. The different phosphate buffers used were pH 5.0, 6.5 and 7.4. The MBCP polymers were degraded into lower molecular weight monomers.
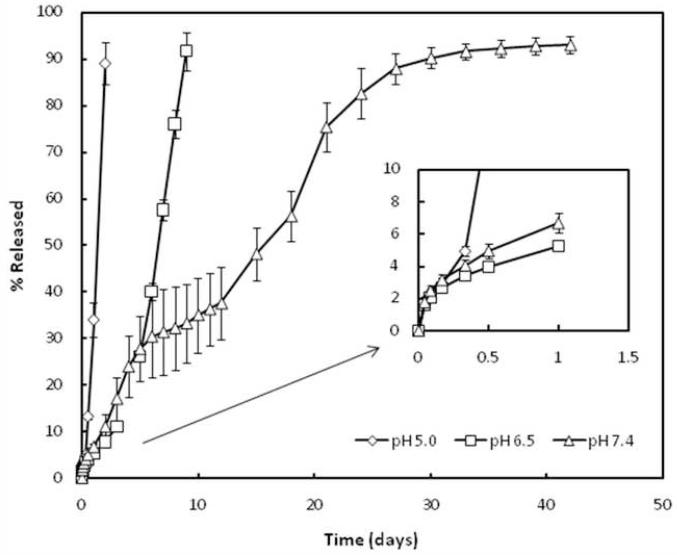
Based on the phase transition study and the dynamic viscosity measurements, MBCP-2 was selected for FITC-Dextran release study. A molecular weight of 40kDa was chosen to simulate the pH-sensitive drug release from the thermogels. The release profiles of FITC-dextran at different pH are shown in Figure 10. FITC-dextran was released for 30 days at pH 7.4. The complete FITC-dextran release was observed within 2 and 9 days at pH 5.0 and 6.5, respectively, which was accordance with the time for gel erosion at each pHs. However, the gels were not eroded even after complete release of FITC-dextran at pH 7.4. The facilitated drug release at lower pH is consistent with the polymer degradation at corresponding pH conditions. FITC-dextran release clearly demonstrates that the release at pH 5.0 is predominantly erosion controlled and the release at pH 6.5 is both diffusion and erosion controlled. In addition, diffusion played main role in the drug release at neutral pH. One unit decrease in the pH of the media caused a pronounced increase in polymer degradation as well as drug release. Likewise, a small change in biological pH occurred by a disease would result in polymer degradation enough for drug release from the polymer gel. These results suggest that the pH of the release medium played a critical role in the drug release from the synthesized polymeric gels, which can be translated into polymeric drug delivery systems that locally administer drugs in response to biological pH changes. The drug delivery system would not be limited to the delivery of small molecule drugs, but would also have applications for sensitive molecules such as proteins, plasmid DNA, and small interfering RNA.
Figure 10.
In vitro release of FITC-Dextran (40 kDa) from MBCP-2 thermogels at pH 5.0, 6.5 and 7.4. FITC-Dextran was dissolved at 10 mg/ml in 25 wt% polymeric aqueous solution. The results represent the mean ± SD for n=5.
4. CONCLUSIONS
Novel biodegradable thermogelling polymers with pH-sensitive acetal linkages have been synthesized from Pluronic® copolymers by a simple method. The synthesized polymers did not cause significant cytotoxicity. The aqueous solutions of these polymers underwent gelation at body temperature and the gelation temperature of the polymer solution was dependent on polymer concentration and the type of Pluronic® copolymer used for the MBCP synthesis. Micellar aggregation was observed for both MBCP polymers at body temperature. MBCP polymers were degraded in a pH dependent fashion. At a neutral pH, polymers were stable for a long period of time while rapid degradation was observed at acidic pHs. FITC-Dextran of molecular weight 40 kDa was released from the thermally-formed polymeric gels in a pH-dependent fashion.
5. ACKNOWLEDGEMENTS
The authors acknowledge the supports from Faculty Research Program (FRP) at The University of Mississippi. We also acknowledge the research facility support from the NIH National Center for Research Resources through C06 RR-14503-01 and P20 RR021929.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- [1].Sun KH, Sohn YS, Jeong B. Thermogelling poly(ethylene oxide-b-propylene oxide) disulfide multiblock copolymer as a thiol-sensitive degradable polymer. Biomacromolecules. 2006;7:2871–2877. doi: 10.1021/bm060512r. [DOI] [PubMed] [Google Scholar]
- [2].Kim S, Healy KE. Synthesis and characterization of injectable poly(n-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4:1214–1223. doi: 10.1021/bm0340467. [DOI] [PubMed] [Google Scholar]
- [3].Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- [4].Booth C, Attwood D. Effects of block architecture and composition on the association properties of poly(oxyalkylene) copolymers in aqueous solution. Macromol Rapid Commun. 2000;21:501–527. [Google Scholar]
- [5].Bhardwaj R, Blanchard J. Controlled-release delivery system for the r-MSH analog melanotan-I using poloxamer 407. J Pharm Sci. 1996;85:915–919. doi: 10.1021/js960097g. [DOI] [PubMed] [Google Scholar]
- [6].Wenzel JG, Balaji KS, Koushik K, Navarre C, Duran SH, Rahe CH, Kompella UB. Pluronic® F127 gel formulations of Deslorelin and GnRH reduce drug degradation and sustain drug release and effect in cattle. J Control Release. 2002;85:51–59. doi: 10.1016/s0168-3659(02)00271-7. [DOI] [PubMed] [Google Scholar]
- [7].Hinrichs WLJ, NME Schuurmans-Nieuwenbroek, van de Wetering P, Hennink WE. Thermosensitive polymers as carriers for DNA delivery. J Control Release. 1999;60:249–259. doi: 10.1016/s0168-3659(99)00075-9. [DOI] [PubMed] [Google Scholar]
- [8].Li Z, Ning W, Wang J, Choi A, Lee PY, Tyagi P, Huang L. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharm Res. 2003;20:884–888. doi: 10.1023/a:1023887203111. [DOI] [PubMed] [Google Scholar]
- [9].Jeong B, Wang LQ, Gutowska A. Biodegradable thermoreversible gelling PLGA-g-PEG copolymers. Chem Commun. 2001;16:1516–1517. [Google Scholar]
- [10].Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, Rhee BG, Mestecky J, Moldoveanu Z, Morgan M, Weitman S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- [11].Ahn JS, Suh JM, Lee M, Jeong B. Slow eroding biodegradable multiblock poloxamer copolymers. Polym Int. 2005;54:842–847. [Google Scholar]
- [12].Jeong B, Bae YH, Kim SW. Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules. 1999;32:7064–7069. [Google Scholar]
- [13].Jeong B, Kibbey MR, Birnbaum JC, Won YY, Gutowska A. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA. Macromolecules. 2000;33:8317–8322. [Google Scholar]
- [14].Hwang MJ, Suh JM, Bae YH, Kim SW, Jeong B. Caprolactonic Poloxamer Analog: PEG-PCL-PEG. Biomacromolecules. 2005;6:885–890. doi: 10.1021/bm049347a. [DOI] [PubMed] [Google Scholar]
- [15].Jo S, Kim J, Kim SW. Reverse Thermal Gelation of Aliphatically Modified Biodegradable Triblock Copolymers. Macromol Biosci. 2006;6:923–928. doi: 10.1002/mabi.200600127. [DOI] [PubMed] [Google Scholar]
- [16].Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sosnik A, Cohn D. Reverse thermo-responsive poly(ethylene oxide) and poly(propylene oxide) multiblock copolymers. Biomaterials. 2005;26:349–357. doi: 10.1016/j.biomaterials.2004.02.041. [DOI] [PubMed] [Google Scholar]
- [18].Jeong B, Lee KM, Gutowska A, An YH. Thermogelling biodegradable copolymer aqueous solutions for injectable protein delivery and tissue engineering. Biomacromolecules. 2002;3:865–868. doi: 10.1021/bm025536m. [DOI] [PubMed] [Google Scholar]
- [19].Determan MD, Cox JP, Seifert S, Thiyagarajan P, Mallapragada SK. Synthesis and characterization of temperature and pH-responsive pentablock copolymers. Polymer. 2005;46:6933–6946. [Google Scholar]
- [20].Huynh DP, Nguyen MK, Pi BS, Kim MS, Chae SY, Lee KC, Kim BS, Kim SW, Lee DS. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials. 2008;29:2527–2534. doi: 10.1016/j.biomaterials.2008.02.016. [DOI] [PubMed] [Google Scholar]
- [21].Schacht E, Toncheva V, Vandertaelen K, Heller J. Polyacetal and poly(ortho ester)—poly(ethylene glycol) graft copolymer thermogels: Preparation, hydrolysis and FITC-BSA release studies. J Control Release. 2006;116:219–225. doi: 10.1016/j.jconrel.2006.07.026. [DOI] [PubMed] [Google Scholar]
- [22].Tomlinson R, Klee M, Garrett S, Heller J, Duncan R, Brocchini S. Pendent chain functionalized polyacetals that display pH-dependent degradation: A platform for the development of novel polymer therapeutics. Macromolecules. 2002;35:473–480. [Google Scholar]
- [23].Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- [24].Leeper DB, Engin K, Thistlethwaite AJ, Hitchon D, Dover JD, Li DJ, Tupchong L. Human tumor extracellular pH as a function of blood glucose concentration. Int J Radiat Oncol Biol Phys. 1994;28:935–943. doi: 10.1016/0360-3016(94)90114-7. [DOI] [PubMed] [Google Scholar]
- [25].Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- [26].Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flower-like micelles of poly(L-lactic acid)-b-poly(ethylene glycol)-b-poly(L-histidine) J Control Release. 2007;23:19–26. doi: 10.1016/j.jconrel.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ojugo AS, McSheehy PM, McIntyre DJ, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- [28].Li YY, Zhang XZ, Cheng H, Kim GC, Cheng SX, Zhuo RX. Novel stimuli-responsive micelle self-assembled from Y-shaped P(UA-Y-NIPAAm) copolymer for drug delivery. Biomacromolecules. 2006;7:2956–2960. doi: 10.1021/bm060080k. [DOI] [PubMed] [Google Scholar]
- [29].Fernández-Carballido A, Pastoriza P, Barcia E, Montejo C, Negro S. PLGA/PEG-derivative polymeric matrix for drug delivery system applications: Characterization and cell viability studies. Int J Pharm. 2008;352:50–57. doi: 10.1016/j.ijpharm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [30].Bhatia SK, Arthur SD. Poly(vinyl alcohol) acetoacetate-based tissue adhesives are non-cytotoxic and non-inflammatory. Biotechnol Lett. 2008;30:1339–1345. doi: 10.1007/s10529-008-9709-2. [DOI] [PubMed] [Google Scholar]
- [31].Heller J, Penhale D, Helwing R. Preparation of polyacetals by the reaction of divinyl ethers and polyols. J Polym Sci Polym Lett Ed. 1980;18:293–297. [Google Scholar]
- [32].Hashimoto T, Ishizuka K, Umehara A, Kodaira T. Synthesis of polyacetals with various main-chain structures by the self-polyaddition of vinyl ethers with a hydroxyl function. J Polym Sci Part A: Polym Chem. 2002;40:4053–4064. [Google Scholar]
- [33].Hashimoto T, Umehara A, Urushisaki M, Kodaira T. Synthesis of a new degradable polyurethane elastomer containing polyacetal soft segments. J Polym Sci Part A: Polym Chem. 2004;42:2766–2778. [Google Scholar]
- [34].Alexandridis P, Holzwarthf JF, Hatton TA. Micellization of Poly(ethy1ene oxide)-Poly(propy1eneoxide)-Poly(ethy1ene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules. 1994;27:2414–2425. [Google Scholar]
- [35].Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- [36].Jeong B, Kim SW, Bae YH. Thermosensitive sol—gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- [37].Hashimoto T, Shibayama M, Kawai H. Ordered structure in block polymer solutions. 4. Scaling rules on size of fluctuations with block molecular weight, concentration, and temperature in segregation and homogeneous regimes. Macromolecules. 1983;16:1093–1101. [Google Scholar]
- [38].Mortensen K, Pedersen JS. Structural study on the micelle formation of poly(ethy1ene oxide)-poly(propy1ene oxide)-poly(ethy1ene oxide) triblock copolymer in aqueous solution. Macromolecules. 1993;26:805–812. [Google Scholar]
- [39].Wanka G, Hoffmann H, Ulbricht W. Phase diagrams and aggregation behavior of poly (oxyethy1ene)-poly (oxypropylene) -poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules. 1994;27:4145–4159. [Google Scholar]
- [40].Cabana A, Ait-kadi A, Juhasz J. Study of the gelation process of polyethylene oxidea—polypropylene oxideb—polyethylene oxidea copolymer (poloxamer 407) aqueous solutions. J Colloid Interface Sci. 1997;190:307–312. doi: 10.1006/jcis.1997.4880. [DOI] [PubMed] [Google Scholar]
- [41].Metters AT, Bowman CN, Anseth KS. A statistical kinetic model for the bulk degradation of PLA-b-PEG-b-PLA hydrogel networks. J Phys Chem B. 2000;104:7043–7049. [Google Scholar]
- [42].Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Release. 2002;78:199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- [43].Nuttelman CR, Henry SM, Anseth KS. Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials. 2002;23:3617–3626. doi: 10.1016/s0142-9612(02)00093-5. [DOI] [PubMed] [Google Scholar]