Abstract
Electrospun fibers have been fabricated for wide use as artificial tissue engineering scaffolds. In particular, fibers smaller than a cell body have been extensively employed to mimic natural extracellular matrix (ECM) and to explore specific responses by various cell types. We investigated the effects of various poly(lactic acid-co-glycolic acid) (PLGA) fiber features on embryonic hippocampal neurons in the early developmental stages in terms of initial axon formation (i.e., polarization) and axon orientation. We produced PLGA fibers that have average diameters ranging from 0.44 µm to 2.2 µm and different degrees of fiber alignment (16–58° in angular standard deviation). After 22 h in culture, embryonic hippocampal neurons grown on PLGA fibers exhibited more axon formation with a 30–50% increase over those on spin-coated smooth PLGA films. This improvement was independent of fiber diameter and alignment; however, slightly more polarization was observed on the smaller fibers and the more aligned fibers. In addition, average axon length of the polarized embryonic hippocampal neurons was not significantly different among the PLGA fibers when compared to cells grown on spin-coated PLGA films. These findings suggest that fibers of subcellular diameters stimulate initial axon establishment and guide the direction of axonal extension; however these fibers do not appear to affect overall axon length. This information will be valuable in understanding the roles of subcellular features on neuron development and for the design of biomaterials for neural tissue interfacing.
Keywords: hippocampal neuron, polarization, electrospinning, PLGA fibers, nerve tissue engineering
1. Introduction
Electrospun fibers have been widely studied for a number of tissue engineering applications because of their attractive features such as high surface area, interconnecting pores, and good mechanical support1,2. In particular, electrospinning techniques have allowed the production of fibers smaller than a cell body and even down to several nanometers, and thus capable of mimicking architectural and topographic features of the extracellular matrix (ECM)1. These small fibers not only provide high surface area, but also present a means to control cellular behaviors, such as adhesion and proliferation. In addition to fiber diameter, fiber alignment has been found to regulate cellular behaviors including contact guidance and differentiation3,4. Electrospinning can be used to create fibers bearing various features and chemical compositions using numerous materials, such as synthetic polymers (e.g., PLGA, polycaprolactone (PCL)) and natural polymers (e.g., collagen, hyaluronic acid)5–8. For cell culture, a number of studies have demonstrated that electrospun fibers are able to support and regulate cell growth and differentiation in vivo and in vitro1,2. For example, nanofibrous poly (L-lactic acid) (PLLA) scaffolds supported adhesion and proliferation of human vein endothelial cells9, and PCL nanofibers induced chondrogenesis of adult bone marrow-derived mesenchymal stem cells10.
For neural tissue applications, Ramakrishna and colleagues demonstrated that electrospun PLLA nanofibers supported neuron growth and differentiation in vivo as well as in vitro4,11. Subsequent studies have also shown that electrospun fibers are suitable and even beneficial for neuron cultures, where growth and differentiation on electrospun fibers were observed for dorsal root ganglion (DRG) neurons, Schwann cells, and cortical neurons12,13. Hence, fibers of various diameters and degrees of alignment create topographical features that influence neuronal behavior. However, little has been explored with respect to the effects of various fibers of micron scales on neural differentiation (i.e., axon formation) and an axonal alignment (i.e., contact guidance) at early developmental stages, of which an understanding will be beneficial for neuroscientists and bioengineers studying nerve tissue repair and regeneration.
To investigate the initial formation of an axon in response to various fiber features, we have used embryonic hippocampal neurons, which have been widely used because of their spontaneous establishment of a highly polarized morphology with a single axon and multiple dendrites14. Also, hippocampal neurons have been reported to show specific responses to topographical features, such as pores and microgrooves 15,16. Hippocampal differentiation in vitro involves five developmental stages17. First, an adherent neuron forms a motile lamellipodium (stage 1). Then, the neuron differentiates by forming multiple neurites (stage 2), followed by establishment of a single axon among the immature neurites (neuron polarization, stage 3) after 24–48 h in culture. This is followed by maturation with further extension of the axon and formation of multiple dendrites (stages 4 and 5). At this stage, the mature hippocampal neurons form synaptic contacts and communicate with other neurons, establishing neural networks. In tissue culture, neuron polarization occurs spontaneously, although the rate of polarization is affected by the cellular environment, including various biochemical molecules such as neurotrophins and calcium ions14,18–20.
Importantly, neuron polarization can be also modulated in tissue culture by non-biochemical signals including mechanical and topographical properties of the biomaterial substrate16,21. In a recent study, we showed that poly(dimethyl siloxane) (PDMS) microchannels of both 1 and 2 µm width promoted polarization of embryonic hippocampal neurons compared to smooth PDMS substrates16. However, while the extent of polarization was similar for 1 µm and 2 µm patterns, axons preferentially crossed the 1 µm ridges, whereas they were aligned along the direction of 2 µm ridges. Based on this previous study, we hypothesize that topographies created by electrospun fibers of micron sizes may play a similar role to the micropatterned substrates in supporting axon initiation and alignment of embryonic hippocampal neurons.
To determine the effects of fiber features in initial neuronal differentiation, PLGA meshes were electrospun with various fiber diameters (0.44, 1.5, and 2.2 µm) and alignments (16, 28, and 53°) (Table 1). Rat embryonic hippocampal neurons were cultured for 22 h on these fibers and on spin-coated PLGA flims, and analyzed using immunostaining of axonal protein (tau-1) to monitor axonal growth (i.e., polarization and extension) and alignment.
Table 1.
PLGA fibers synthesized and tested for embryonic hippocampal neuron culture
| Synthesis Conditions | Analysis | |||||
|---|---|---|---|---|---|---|
| Samples | Concentration (wt % PLGA) |
Drum Speed (m/s) |
No. Fibers Analyzed (n) |
Average Diameter ± STD*(µm) |
Angular Standard Deviation (°) |
Fiber Orientation |
| RD_7 | 7 | 0 | 210 | 0.44 ± 0.18 | 57.84 | Random |
| RD_10.5 | 10.5 | 0 | 167 | 1.51 ± 0.56 | 52.93 | Random |
| IN_10.5 | 10.5 | 4.9 | 233 | 1.25 ± 0.52 | 27.67 | Intermediate |
| HI_10.5 | 10.5 | 10 | 189 | 0.97 ± 0.32 | 15.89 | High |
| RD_13 | 13 | 0 | 115 | 2.22 ± 0.55 | 50.07 | Random |
STD*=standard deviation
2. Materials and Methods
2.1 Electrospinning
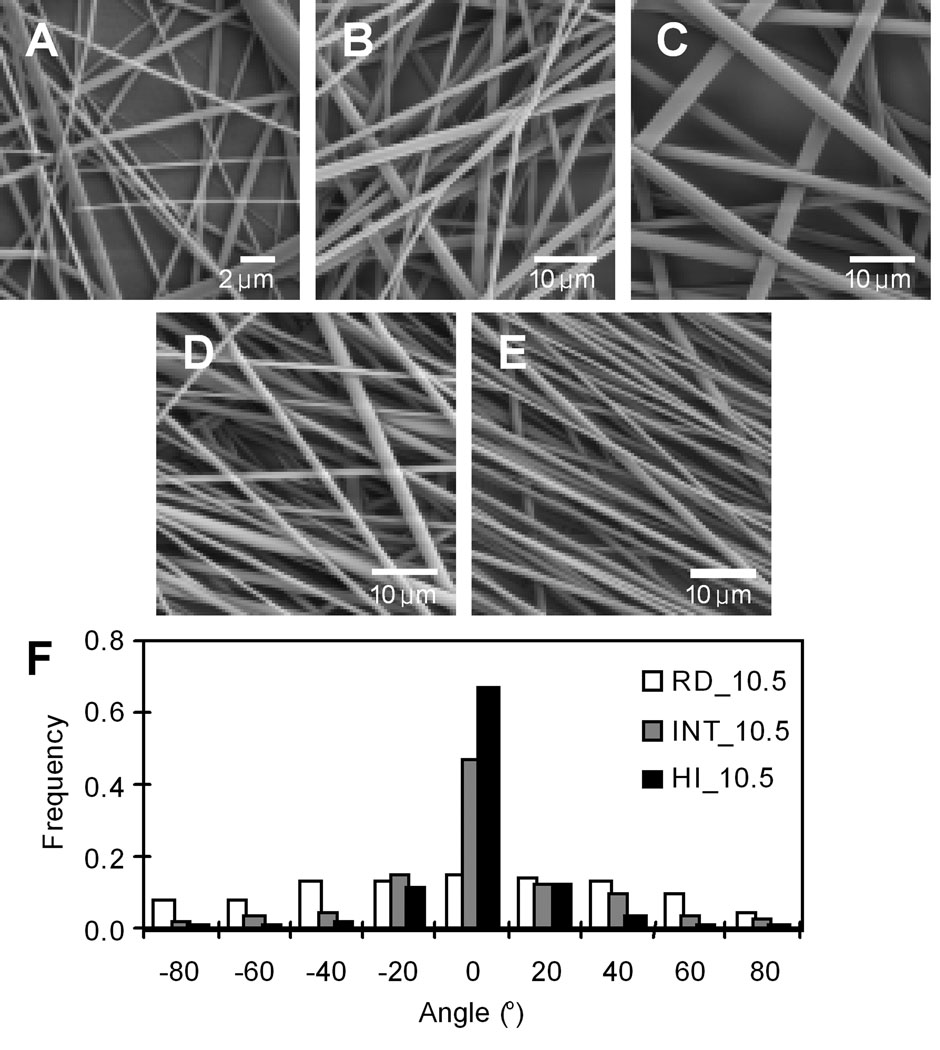
A 75/25 poly(D,L lactic-co-glycolic acid) (PLGA) (inherent viscosity 0.55–0.75 dL/g) was purchased from Lactel Biodegradable Polymers (Birmingham, AL) and electrospun onto 18 mm circular glass coverslips (Sigma, St. Louis, MO) to form fused-fiber meshes with controlled fiber diameters and degrees of fiber alignment as described previously\22. Briefly, glass coverslips were sonicated in ethanol and allowed to air dry. Next, a 0.30 mL volume of a 3.5 wt% solution of PLGA in dichloromethane (Sigma) was deposited with a Model 1-EC101D-R485 spincoater (Headway Research, Garland, TX) onto the glass to form a smooth spin-coated polymer film. The spincoater was operated at a rotational speed of 2,500 rpm for 30 s, and the samples were allowed to air dry. For fiber scaffolds, the spin-coated coverslips were then mounted onto a stationary stand and PLGA was electrospun under ambient conditions to form fiber meshes with random fiber orientation. Fiber diameter was controlled by using PLGA concentration in hexafluoro-2-propanol (HFIP) (Sigma) of 7.0, 10.5, and 13.0 wt%. Electrospinning was performed with a syringe equipped with a 22 gauge Teflon-tipped needle using a 15 kV potential, a throw distance of 15 cm, and a syringe flow rate of 5 mL/h. To form oriented meshes, the spin-coated coverslips were mounted onto a 7.6 cm diameter drum rotated at linear velocities of 4.9 and 10.0 m/s and PLGA was electrospun. After electrospinning, PLGA coverslips were air dried for 2 days to remove residual HFIP. Representative SEM images of PLGA fibers on coverslips are displayed in Figure 1.
Figure 1.
Scanning electron micrographs of PLGA fibers electrospun using different concentrations and rotation speeds of a collector: Random fibers of (A) RD_7, (B) RD_10.5, and (C) RD_13 were electrospun using 7%, 10.5%, and 13% polymer solution at a stationary mode, respectively. Aligned fibers were produced from 10.5% concentration on a rotating collector: (B) 0 m/s (random fibers, RD_10.5), (D) 4.9 m/s (intermediately oriented fibers, INT_10.5), and (E) 10 m/s (highly oriented fibers, HI_10.5). (F) Normalized histograms of fiber angles for the fibers (B, D, and E). At least 100 individual fibers were analyzed from a representative sample for each substrate.
2.2 Hippocampal Cell Culture
For cell culture experiments, electrospun meshes and spin-coated films were transferred to a 12-well cell culture plate (BD, Franklin Lakes, NJ), and sterilized by exposure to UV for 2 h. The samples were incubated in 0.2 mg/mL poly-D-lysine (Sigma) overnight, and washed twice with sterile double deionized (ddI) water. The substrates were dried in a laminar flow bench, and stored at 4°C until use. Rat embryonic hippocampal neurons (E-18) were isolated from commercial rat hippocampal tissue (BrainBits, Springfield, IL) according to the manufacture’s protocol. In brief, a hippocampus was incubated in 4 mg/mL papain (Worthington, Lakewood, NJ) solution in Hibernate E medium (BrainBits) at 30°C for 20 min. A fire-polished Pasteur pipette was used to triturate the hippocampal tissue, followed by centrifugation (200 g, 1 min). The cell pellet was suspended in 1 mL of warm culture medium, containing Neurobasal medium (Invitrogen, Gaithersburg, MD), 2% B-27 supplement (Invitrogen), 0.5 mM L-glutamine (Fisher, Pittsburgh, PA), 0.025 mM glutamic acid (Sigma), and 1% antibiotic-antimycotic solution (Sigma). 2 × 104 cells/cm2 were inoculated onto different samples of PLGA fibers and spin-coated coverslips. The cells were incubated at 37°C in a humid, 5% CO2 incubator for 22 h. For each substrate, data were collected from at least five experiments performed on separate days.
2.3 Scanning Electron Microscopy (SEM)
SEM was used to analyze electrospun PLGA fibers and hippocampal neurons on the substrates for cellular morphologies and interaction with fibers. For the characterization of the fibers, high resolution images of electrospun meshes were acquired using a LEO1550 Field Emission SEM (Carl Zeiss SMT, Thornwood, NY). Briefly, samples were mounted onto studs and sputtercoated with 20 nm of palladium using a Cressington Scientific Instruments Model 208HR (Cranberry Township, PA).
For cell images, the fixed hippocampal neurons were dehydrated by successive treatment with increasing ethanol concentrations in water (30% for 45 min; 50% for 30 min, 70%, 85%, 90%, 95%, and absolute ethanol (Pharmco, Brookfield, CT) for 10 min each). Water was completely removed by adding hexamethyl disilazane (HMDS) (Sigma) and drying in air. The dried samples were coated with a 10 nm thick layer of platinum/palladium using a sputter coater (Cressington 208HR). SEM images were acquired with a Zeiss SUPRA 40 VP Scanning Electron Microscope.
2.4 Fiber Characterization
The diameter and degree of orientation of the electrospun fibers were measured from already defined SEM images using ImagePro Plus software (ICube, Crofton, MD) as previously described22. At least 100 individual fibers were measured from a representative sample for each substrate. The degree of fiber alignment was characterized by a wrapped normal distribution and expressed as angular standard deviation (ASD), in which a smaller value of ASD indicates a greater alignment of the individual fibers. Histograms of individual fibers were plotted (relative to the mean) by adjusting for a period of −90° to 90°.
2.5 Immunofluorescence
Embryonic hippocampal neurons cultured on the PLGA substrates were fixed with 4% paraformaldhyde (Sigma) and 4% sucrose (Sigma) in phosphate buffered saline (PBS, pH 7.2) for 20 min at room temperature. Fixed samples were permeabilized with 0.1% Triton X-100 (Fluka, St. Louis, MO) and 3% goat serum (Sigma) in PBS buffer for 20 min, washed twice with PBS, and treated with blocking solution of 3% goat serum in PBS for 1 h at 37°C. Tau-1, a microtubule protein expressed in axons, was labeled as an axonal marker23. Mouse tau-1 antibody (Chemicon, Temecula, CA) was diluted to 1:200 in blocking solution, and added to the samples. After overnight incubation at 4°C, the samples were washed with PBS two times, and treated with a secondary antibody solution of Alexa 488-labeled goat anti-rat IgG (Invitrogen) (1:200 dilution in blocking solution) at 4°C for 5 h, followed by two PBS washes (5 min, each) and storage at 4°C until analysis.
Fluorescence images of cells and axons were acquired using a fluorescence microscope (IX-70, Olympus, Center Valley, PA). Ten to twenty images were randomly captured per sample using a color CCD camera (Optronics MagnaFire, Goleta, CA). The cell images were analyzed using Image J (NIH) software. Axon length was measured as a linear distance between the cell junction and the tip of an axon. When axons were branched from a single neuron, the longest axon was measured. Also, a neuron was considered to be polarized only when the axon was two times longer than the cell body16,17. The fraction of polarized neurons on each substrate was calculated from the total cell number and reported. Axon alignment was also analyzed in an analogous manner as that for fiber alignment. The orientation of each axon (the straight line from the axonsoma junction to the end of the axon) was measured relative to the vertical direction of the sample and then ASD was calculated from the resultant angles.
2.6 Statistics
The percentage of polarized neurons and average axon lengths were calculated from analysis of at least 100 neurons per substrate. Experiments were repeated on different days and the data were reported as the mean ± standard deviation for n≥5 independent substrates. Statistical significance was calculated using a Student’s t-test with Origin software (MicroCal, Northampton, MA) and the criterion for statistical significance was p<0.05. Also, axonal alignment was determined using at least 100 axons from a representative sample for each substrate.
3. Results
3.1 Fabrication of Electrospun PLGA Fibers
We fabricated various PLGA fibers having diameters smaller than a neuron cell body (~15 µm) by controlling polymer concentrations of PLGA (Table 1). We obtained PLGA fibers of 2.22 ± 0.55, 1.51 ± 0.56, and 0.44 ± 0.18 µm in diameter by simply employing different PLGA concentrations (13% (w/w), 10.5%, and 7%, respectively) (Figure 1). High concentration of the PLGA solution increases the number of chain entanglements. This leads to increased solution viscosity and larger average diameter of the PLGA fibers when the solution is electrospun1.
Production of differently aligned fibers was achieved by varying the rotation speed of a collector drum (Figure 1). PLGA was electrospun using 10.5% (w/w) polymer solution at different rotation speeds of 0 m/s, 4.9 m/s, and 10 m/s, to produce randomly aligned fibers (RD_10.5), intermediately aligned fibers (INT_10.5), and highly aligned fibers (HI_10.5), respectively (Table 1). A smaller ASD indicates a narrower distribution of fiber orientations, and thus more alignment of the fibers; whereas a higher value is closer to random orientation. At a higher rotating speed, fibers exhibited a smaller diameter (0.97 ± 0.32 µm) compared to those spun at lower speed or on a stationary stage (1.25 ± 0.52 µm in INT_10.5 and 1.51 ± 0.56 µm in RD_10.5) even with the same polymer concentration (10.5%). This trend may be attributed to the stretching of fibers by the rotating collector drum, and has been reported by other groups4,12,22.
3.2 Effects of Fiber Sizes
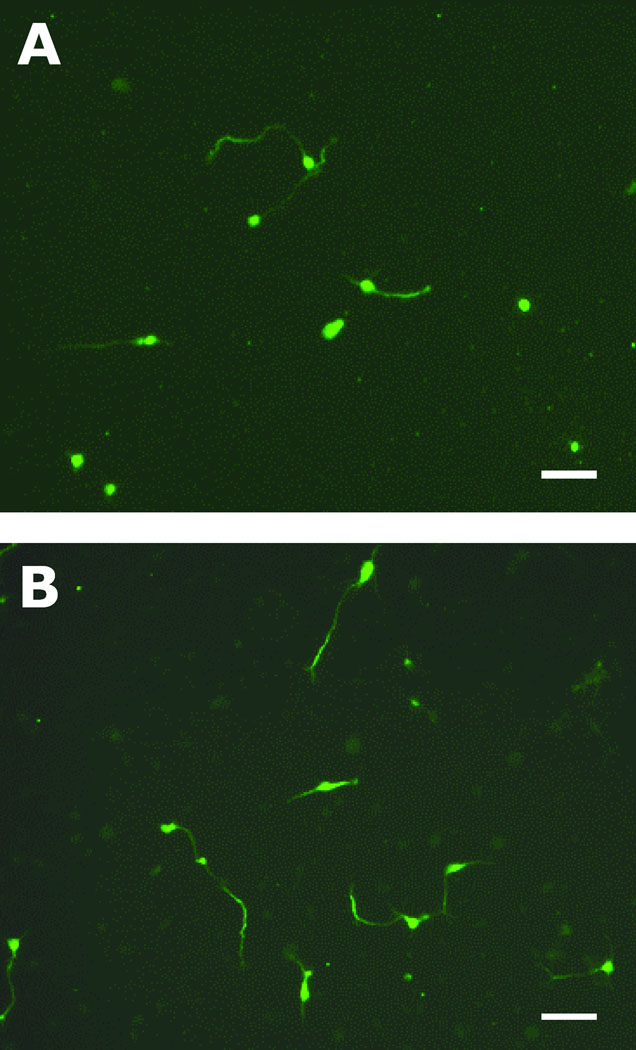
Rat embryonic hippocampal neurons were cultured on PLGA fibers having different fiber sizes and spin-coated substrates as controls. After 22 h, axon formation and length were analyzed on each substrate. Because tau-1 is widely used as an axonal marker, immunostaining for tau proteins allowed investigation of the axonal development process of embryonic hippocampal neurons. Figure 2 shows representative tau-1 immunostaining images of hippocampal neurons grown on the spin-coated PLGA substrate and PLGA nanofibers (RD_7) after 22 h in culture.
Figure 2.
Immunostaining of hippocampal neurons on (A) a spin-coated PLGA films and (B) PLGA nanofibers (RD_7). After 22 h in culture, the cells were fixed using para-formaldehyde, and stained for tau-1 (axonal marker). Scale bars are 50 µm.
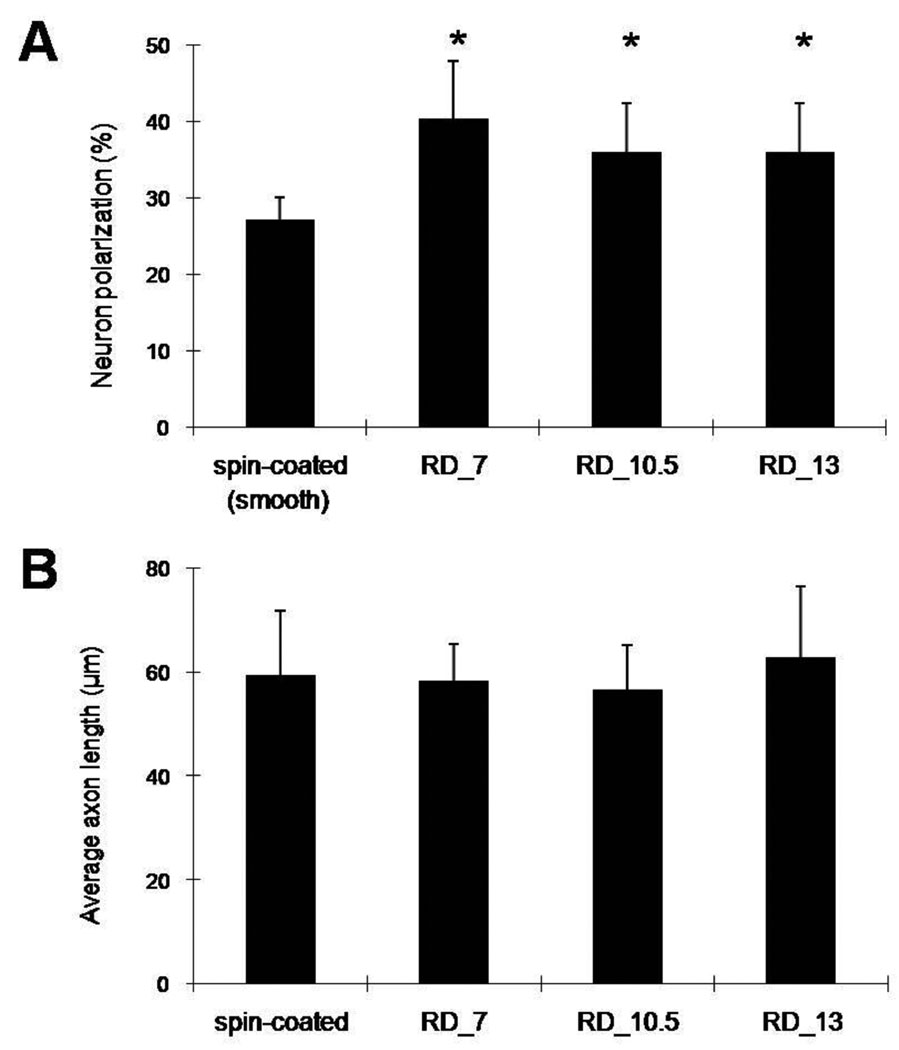
More axon establishment was observed on PLGA fibers than on spin-coated controls. Figure 3A shows that 35–40% of hippocampal neurons polarized on the fibers, which is significantly different from controls (27%) (p<0.05). We observed a trend that the smallest PLGA fibers (RD_7) promoted greater polarization of hippocampal cells compared to larger fibers (RD_10.5 and RD_13); however, the differences were not statistically significant. Fiber diameters did not affect the overall average axon length of polarized neurons (Figure 3B).
Figure 3.
Effects of fiber diameter on neuron polarization and average axon lengths of polarized hippocampal neurons cultured for 22 h. (A) Polarization of rat embryonic hippocampal neurons on spin-coated substrates (controls) and different PLGA fibers (0.44, 1.51, and 2.22 µm for diameters of RD_7, RD_10.5, and RD_13, respectively). Polarization of hippocampal neurons was significantly higher on all PLGA fibers than on smooth spin-coated PLGA substrates; however, no significant difference in neuron polarization was observed among PLGA fibers. (B) Fiber diameters and fibrous topographies did not significantly affect axonal length among the samples. Each bar represents the average ± the standard deviation from six experiments (n=6), and an asterisk denotes a statistically significant difference relative to cells on spin-coated substrates (p<0.05).
3.3 Effects of Fiber Orientation
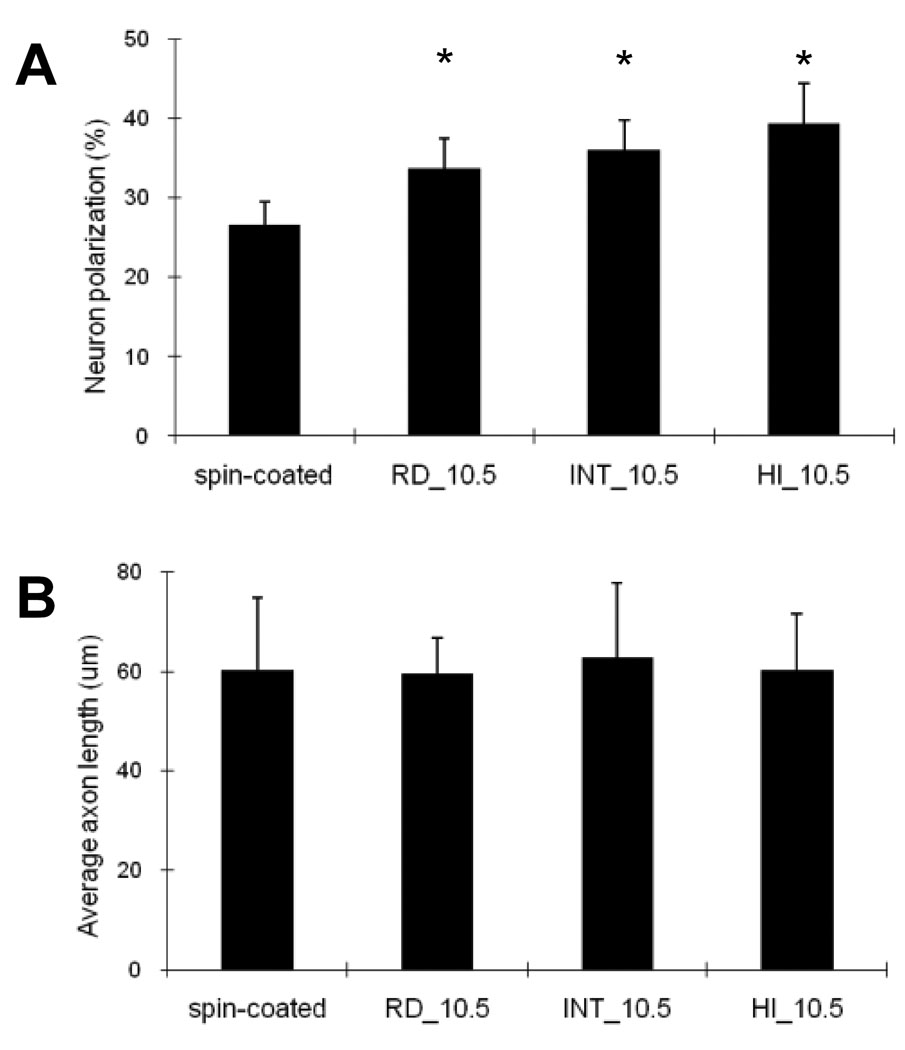
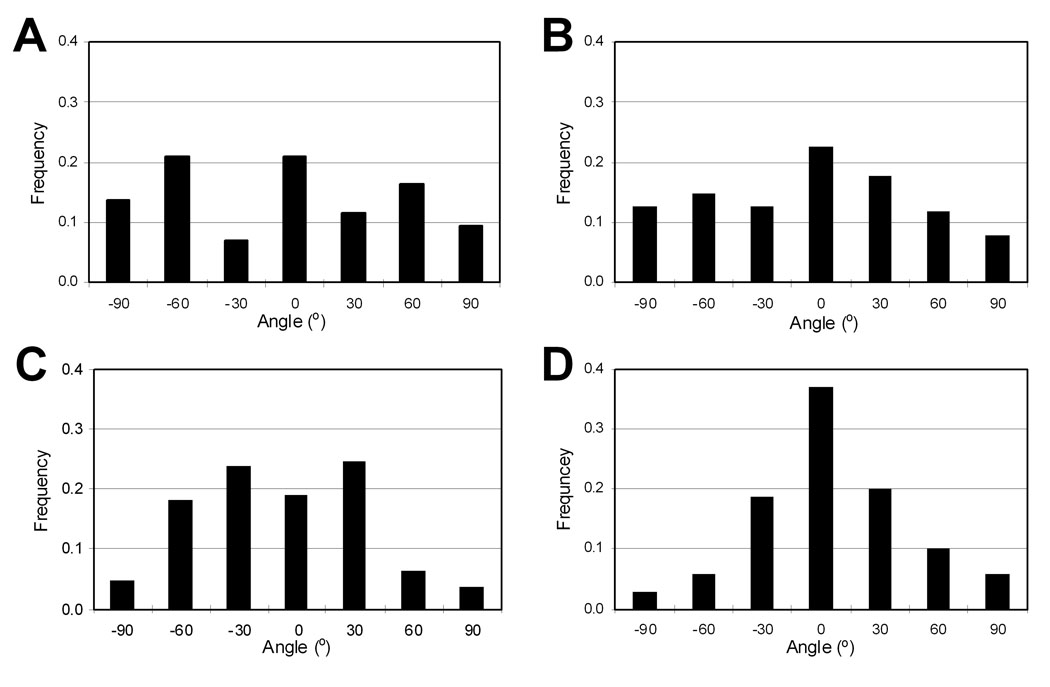
We investigated the effects of fiber orientation on embryonic hippocampal neurons using differently oriented PLGA fibers (RD_10.5, INT_10.5, and HI_10.5) and spin-coated controls. As shown in Figure 4A, after 22 h in culture, hippocampal neurons cultured on the fibers established significantly more axons (34–40%) than neurons on spin-coated controls (p<0.05). No significant difference was observed within the fiber samples, indicating that fiber orientation had negligible effects on axonal establishment within these size ranges. However, an overall trend of larger numbers of cells polarized on the more oriented fibers was observed. In addition, similar to the results described in Section 3.2, overall axon length was not different among the cells cultured on the oriented fibers and controls (Figure 4B). Fiber orientation itself does not appear to considerably influence axonal formation and elongation of embryonic hippocampal neurons. Immunofluorescence images of hippocampal neurons in Figure 5 show axonal alignment in the major direction of fibers. Neurons extended their axons along fibers, which resulted in a narrower axonal angle distribution (Figure 6). Axons mostly were found to grow along the fiber strands and often crossed over to other fibers but kept following the new strands near fiber junctions, which are indicated by arrows in Figure 5. Therefore, the ability to guide axons in a certain direction appears to be proportional to the degree of fiber orientation. As seen in Figure 6, the polarized neurons on the spin-coated substrates and random PLGA fibers (RD_10.5) extended their axons in random directions, resulting in a broad distribution of axon angles, whereas narrower ASDs were observed on the more aligned fibers (INT_10.5 and HI_10.5). ASDs of the hippocampal axons cultured were 27.5°, 33.9°, 71.0°, and 70.4° on HI_10.5, INT_10.5, RD_10.5, and spin-coated controls, respectively. These axon distributions were slightly broader than the fiber orientations (Table 1), but close to them, which suggests that micron scale fibers were able to guide the orientation of the axons.
Figure 4.
Effects of fiber alignment on neuronal polarization and elongation of hippocampal neurons. (A) Polarization on controls and on differently oriented PLGA fibers (RD_10.5, INT_10.5, and HI_10.5). The fibers of different alignments increased neuron polarization compared to the controls. However, the increase in polarization was independent of the degree of fiber alignment, resulting in similar polarization of hippocampal neurons cultured on PLGA fibers. (B) Average axon lengths of hippocampal neurons cultured on differently oriented PLGA fibers and controls. Results suggest that orientations of micron-scaled fibers did not influence initial axon establishment and axon elongation, whereas fibrous meshes enhanced axon establishment of neurons compared to the smooth controls. Each bar represents the average ± the standard deviation of the means from five experiments (n=5), and an asterisk denotes a statistically significant difference relative to cells on spin-coated substrates (p<0.05).
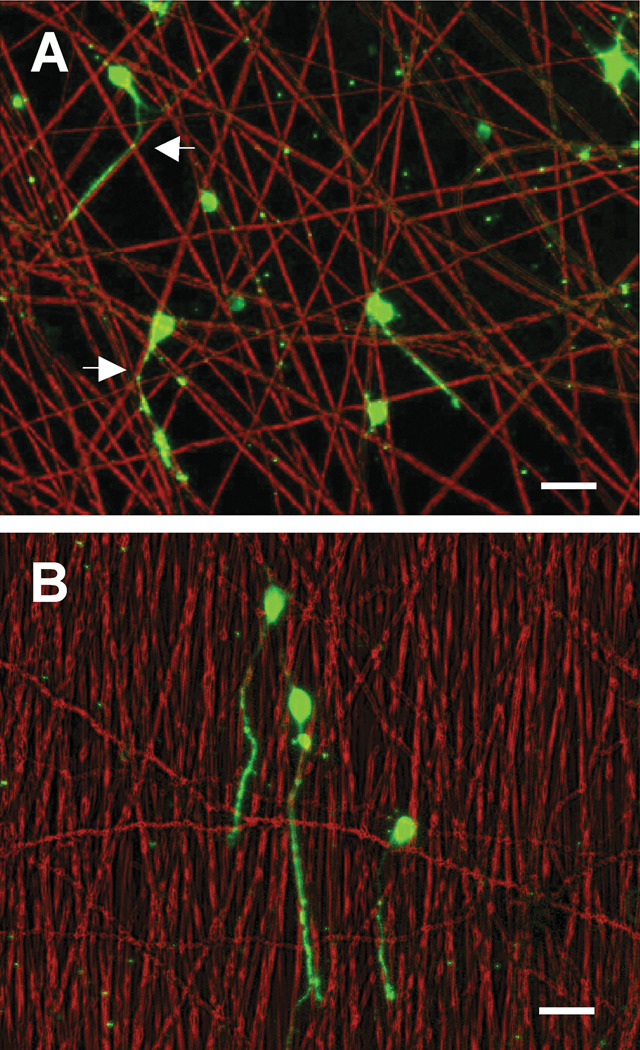
Figure 5.
Hippocampal neurons cultured on (A) random fibers (RD_10.5) and (B) aligned fibers (HI_10.5). Images were constructed by merging phase contrast (red) and immunofluorescence (green) images. Axons on the fibers were observed to grow mostly along the fibers and often to change directions from one fiber to a closely adjacent fiber (arrows indicate changes near fiber junctions). Scale bars are 50 µm.
Figure 6.
Normalized histograms of axon angles on various substrates: (A) the spin-coated films (controls), (B) randomly aligned fibers (RD_10.5), (C) intermediately aligned fibers (INT_10.5), and (D) highly aligned fibers (HI_10.5). At least 100 axons were analyzed from a representative sample for each substrate. Mean values of axon angle on each substrate were adjusted to 0°.
4. Discussion and Conclusion
Micron and submicron scale features can be employed for in vitro and in vivo cell culture to mimic the natural cellular surroundings of the extracellular matrix for various biomedical applications including nerve tissue regeneration1,2. We are interested in the roles of topographical features of fiber meshes on neuron polarization for the purpose of improved understanding and thus improved design of biomaterials for neural applications. Therefore, in this article, we examined the effects of electrospun fiber diameter and alignment on initial axon establishment of embryonic hippocampal neurons.
We previously studied the polarization of embryonic hippocampal neurons on well-defined microgrooves and found that topographical patterns smaller than a soma have the unique ability to induce axon initiation over other biological cues; however, these microgrooved features had little effect on overall axon length16,24. Hippocampal neurons cultured on PDMS microchannels (1–2 µm distance between ridges, 1–2 µm width, 0.3–0.6 µm depth) polarized approximately two times faster than neurons on smooth PDMS samples16. Also, previous studies with other patterned materials have shown similar effects, suggesting that micron-scaled topography is a dominating factor. Specifically, increased neuronal polarization was observed on polypyrrole microchannels compared to non-patterned polypyrrole substrates24. These previous studies emphasized the importance of micron-scaled topography in axon formation of hippocampal neurons. Our results in this study were consistent with these previous findings. The PLGA fibers, ranging from 0.4 µm to 2.2 µm, promoted neuron polarization, but did not have an effect on overall axon elongation after 22 h in culture. In addition, the degree of fiber alignment did not significantly influence neuron polarization. These findings suggest that fibrous features of the tested PLGA samples mainly influence the early developmental stage (axon formation). Overall, the increases in the polarization of the cells on fibrous PLGA substrates (30–50% increase over smooth controls) were not as dramatic as the increases observed on PDMS microgrooves (~100% increase over smooth controls). These differences might result from the irregular patterns of electrospun fibers leading to larger variance in local topographies, compared to precise lithographic microchannels.
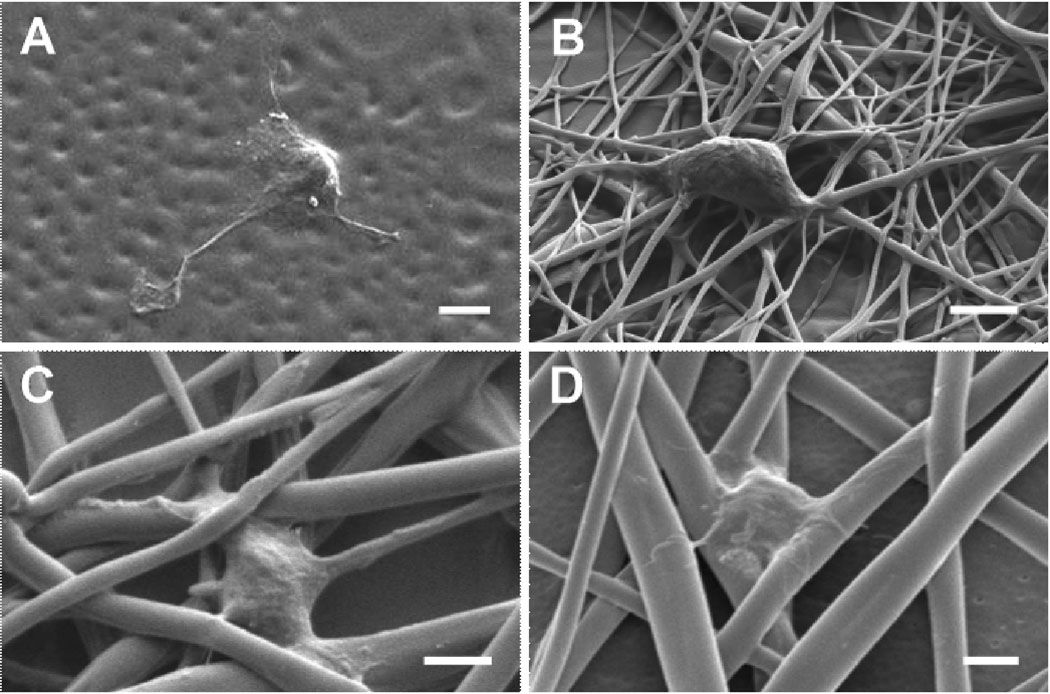
Regarding topographical roles in regulating neuronal behaviors, little is known; however, surface topographical features (i.e., porous, grooved, and fibrous structures) interact differently with cells compared to smooth surfaces, influencing adhesion, migration, and differentiation25–27. Neurons appear to sense subtle nanofeatures on surfaces. For example, PDMS replicas of living Schwann cells and the nanometer-scale features of these cells induced the adhesion and orientation of DRG neurons26. Similarly, human mesenchymal stem cells cultured on nano-patterned PDMS substrata were shown to exhibit selective differentiation into the neuronal lineage compared to the same cells cultured on micro-patterned and smooth substrata27. In the present studies, we postulate that subcellular-scale fibrous features may provide cells under development (stage 2) with topography and different stresses in a similar way to micropatterns. The scanning electron micrographs (Figure 7) illustrate that hippocampal neurons grew mostly on the fibers and interacted with two or more PLGA strands on top of the meshes, suggesting that fibrous topographies of subcellular scales can exert an influence on neurons and neurites/axons. The effects of material topography on cells are not clearly understood. However, one theory is that topographical features in the form of elevation changes facilitate reorganization of focal adhesion complexes (FAC), which mediate cytoskeletal tension, activation of signal transduction, and varied gene expression and phenotypic responses28–30. Presumably, this proposed mechanism may explain the improved polarization of cultured hippocampal neurons on PLGA fibers compared to smooth surfaces in the present studies.
Figure 7.
SEM images of hippocampal neurons cultured on various PLGA meshes: (A) RD_7, (B) RD_10.5, (C) HI_10.5, and (D) RD_13. Scale bars are 2 µm.
Axon alignment has been widely studied on various grooves and ridges of different substrates16,31,32. Axons and neurites dynamically sense local topographies, allowing the cells to adjust their morphologies and orientation. Other research has shown that hippocampal neurons extend their axons preferentially along wider and deeper ridges while crossing over narrower and shallower patterns, indicating that both parameters are effective for guiding axon growth along the grooves16,31,32. Interestingly, we found that most axons of hippocampal neurons cultured on PLGA fibers were aligned along the fibers regardless of the sizes and orientations of the fibers tested. Our analysis of axon orientation indicated that fiber orientation directly promoted axonal alignment (Figure 5). We found that axons on the random fibers (RD_10.5) often changed to another fiber strand near the crossover points of fibers, which supports the idea that the axons of hippocampal neurons can pass over very small gaps at the fiber junctions.
We fabricated the differently oriented fibers to study the effects of the degree of fiber alignment. The range of fiber sizes was 0.97 to 1.51 µm. This size is well within the range of 0.44 µm and 2.2 µm over which fiber diameter had little effect on neuron polarization and axon length (Figure 3). Thus, the degree of fiber alignment, and not fiber diameter, appears to be the key factor influencing axon formation and elongation among these differently oriented fibers. We observed increased axon establishment on more aligned fibers; however, it was not significant. Overall axon length was insensitive to fiber orientation (Figure 4). Other researchers have also performed studies to assess the effect of fiber alignment on neurite length and guidance. Aligned PLLA nanofibers caused elongated shapes of cell bodies, and facilitated neurite elongation of immortalized NSCs with an increase of 25% compared to randomly oriented nanofibers4. Corey et al. cultured DRG explants on differently aligned PLLA nanofibers (524±305 nm in diameter) and found that the aligned PLLA fibers induced neurite alignment and elongation after 3 days in culture12. They assumed that the smaller angle between two adjacent fibers in a highly aligned mesh of nanofibers enabled the growth cone to make a decision to select one fiber to follow, resulting in a longer neurite. However, this trend was not observed in our current studies. The different effects of aligned fibers on neuronal behaviors observed by us and other groups may result from inherent differences between embryonic hippocampal neurons and immortalized NSCs and DRG neurons. Also, the short culture time (22 h) used in our studies may not be sufficient to analyze axon elongation after polarization. Although we have not tested other ranges of fiber sizes, oriented fibers of larger or smaller diameters may result in different responses of hippocampal neurons. Also, the degree of fiber alignment may play a synergistic role in neuronal differentiation in combination with other factors, such as ECM proteins and neurotrophins.
For future studies, long-term culture of hippocampal neurons on various electrospun fibers may be useful to explore axon extension and synaptic formation among neurons. Also, tailoring electrospun fibers with bioactive molecules, such as neurotrophins and cell adhesive molecules (i.e., laminin, fibronectin) would be intriguing to understand the combined effects on neurons and to develop better scaffolds for biomedical applications including nerve tissue regeneration scaffolds.
This work may aid an understanding of the topographical effects on neurons during early development stages. In addition, this study on specific interactions between neurons and fibrous meshes will allow us to better design the surface of biomaterials for neural applications, such as nerve tissue engineering scaffolds and neural prostheses.
Acknowledgment
This work was supported by NIH R01EB004429 (CES) and Institute for Critical Technologies and Sciences at Virginia Tech (ASG). The authors would like to thank Maeve Cooney for proof reading the manuscript.
References
- 1.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 2.Murugan R, Ramakrishna S. Nano-featured scaffolds for tissue engineering: a review of spinning methodologies. Tissue Eng. 2006;12:435–447. doi: 10.1089/ten.2006.12.435. [DOI] [PubMed] [Google Scholar]
- 3.Shin M, Ishii O, Sueda T, Vacanti JP. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials. 2004;25:3717–3723. doi: 10.1016/j.biomaterials.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 4.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 6.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y, Ghosh K, Shu XZ, Li B, Sokolov JC, Prestwich GD, Clark RA, Rafailovich MH. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials. 2006;27:3782–3792. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–328. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 9.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res A. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 10.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Bini TB, Gao S, Tan TC, Wang S, Lim A, Hai LB, Ramakrishna S, et al. Electrospun poly(L-lactide-co-glycolide) biodegradable polymer nanofibre tubes for peripheral nerve regeneration. Nanotechnology. 2004;15:1459–1464. [Google Scholar]
- 12.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, Martin DC. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 2007;83(3):636–645. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 13.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Fukata Y, Kimura T, Kaibuchi K. Axon specification in hippocampal neurons. Neurosci Res. 2002;43(4):305–315. doi: 10.1016/s0168-0102(02)00062-7. [DOI] [PubMed] [Google Scholar]
- 15.Sapelkin AV, Bayliss SC, Unal B, Charalambou A. Interaction of B50 rat hippocampal cells with stain-etched porous silicon. Biomaterials. 2006;27:842–846. doi: 10.1016/j.biomaterials.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Gomez N, Lu Y, Chen S, Schmidt CE. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28:271–284. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26(42):10626–10630. doi: 10.1523/JNEUROSCI.3824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labelle C, Leclerc N. Exogenous BDNF, NT-3 and NT-4 differentially regulate neurite outgrowth in cultured hippocampal neurons. Brain Res Dev Brain Res. 2000;23:1–11. doi: 10.1016/s0165-3806(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 20.Ziv NE, Spira ME. Induction of growth cone formation by transient and localized increases of intracellular proteolytic activity. J Cell Biol. 1998;140(1):223–232. doi: 10.1083/jcb.140.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamoureux P, Ruthel G, Buxbaum RE, Heidemann SR. Mechanical tension can specify axonal fate in hippocampal neurons. J Cell Biol. 2002;159(3):499–508. doi: 10.1083/jcb.200207174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashur CA, Dahlgren LA, Goldstein AS. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27:5681–5688. doi: 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez N, Lee JY, Nickels JD, Schmidt CE. Micropatterned Polypyrrole: Combination of Electrical and Topographical Characteristics for Stimulation of Cells. Adv Func Mater. 2007;17:1645–1653. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan YW, Cui FZ, Hou SP, Xu QY, Chen LN, Lee IS. Culture of neural cells on silicon wafers with nano-scale surface topograph. J Neurosci Methods. 2002;120:17–23. doi: 10.1016/s0165-0270(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 26.Bruder JM, Lee AP, Hoffman-Kim D. Biomimetic materials replicating Schwann cell topography enhance neuronal adhesion and neurite alignment in vitro. J Biomater Sci Polym Ed. 2007;18:967–982. doi: 10.1163/156856207781494412. [DOI] [PubMed] [Google Scholar]
- 27.Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 29.Rajnicek AM, McCaig CD. Guidance of CNS growth cones by substratum grooves and ridges: effects of inhibitors of the cytoskeleton, calcium channels and signal transduction pathways. J Cell Sci. 1997;110:2915–2924. doi: 10.1242/jcs.110.23.2915. [DOI] [PubMed] [Google Scholar]
- 30.Dalby MJ. Topographically induced direct cell mechanotransduction. Med Eng & Phys. 2005;27:730–742. doi: 10.1016/j.medengphy.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Rajnicek AM, Britland S, McCaig CD. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. J Cell Sci. 1997;110:2905–2913. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- 32.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108(4):635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]