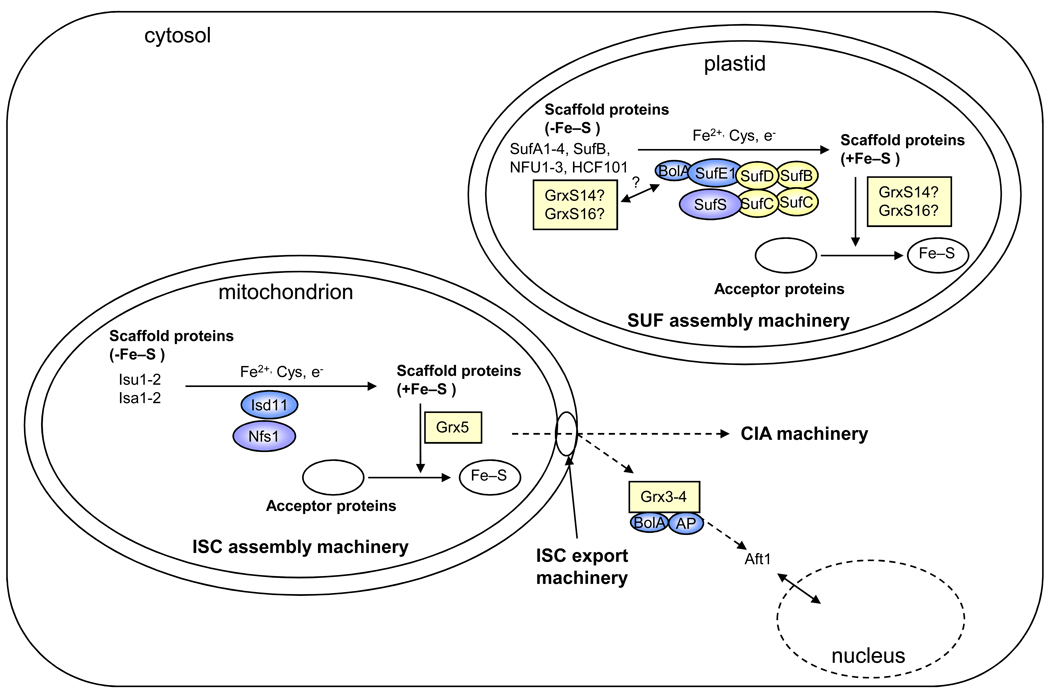
Figure 1. [sc1] Simplified view of the putative Grx roles in Fe–S cluster assembly machineries in eukaryote organelles.
This scheme has been drawn based on the current models of ISC assembly in yeast mitochondria and of the plant plastidial and bacterial SUF assembly machineries [1,6,8,64]. The SUF system is composed of a SufBCD (color) and SufSE (blue and purple) complexes. The nomenclature of Arabidopsis thaliana members has been used for the plastidial model. In mitochondria, Grx5p (color) is most likely involved in Fe–S cluster (color) transfer from Isu1p to acceptor proteins. As orthologs exist in most living organisms and as they generally complement the yeast mutant strain, it is tempting to speculate that this function is conserved among kingdoms. Isu and Isa correspond to U- and A-type scaffold proteins, respectively, Nfs1p (purple) to the cysteine desulphurase and Isd11p (blue) to a protein required for Nfs1p activity. In plastids, it is hypothesized that GrxS14 and GrxS16 (color) can perform scaffolding functions, participate in cluster trafficking between other identified scaffolds (SufA1-4, SufB, NFU1-3, HCF101) and recipient proteins in a manner analogous to the mitochondrial Grx5p, or regulate SUF function through an interaction with the BolA domain of SufE1. Notably, these roles are not mutually exclusive. To complete this view, the CIA (cytosolic iron–sulphur cluster assembly) and ISC (iron–sulphur cluster) export machineries are shown, and the current model for the role of cytosolic Grx3p and Grx4p (color) in iron sensing is depicted. AP: aminopeptidase P-like protein.