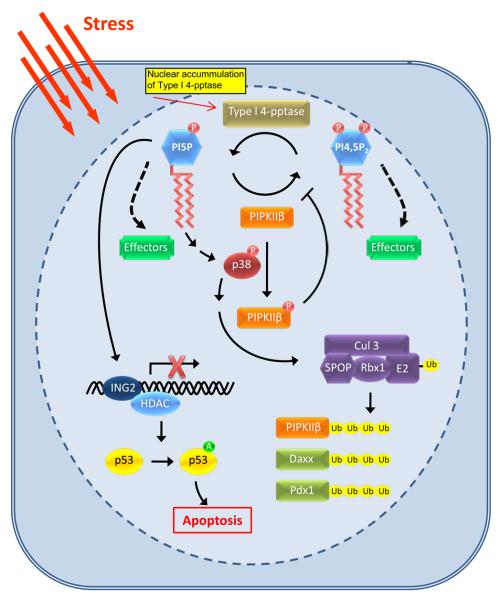
Figure 2.
A nuclear phosphoinositide-mediated stress response pathway. Under resting conditions, PIPKIIβ controls PI5P levels by its synthesis of PI4,5P2. Upon cellular stress, PIPKIIβ activity is attenuated via type 1 PI4,5P2 4-phosphatase and p38 MAPK activity resulting in the accumulation of PI5P. Specifically, in response to cellular stress, such as oxidative stress or UV irradiation, type 1 PI4,5P2 4-phosphatase translocates to the nucleus where it hydrolyzes PI4,5P2 into PI5P. Concurrently, PIPKIIβ is phosphorylated by activated p38 MAPK, inhibiting its lipid kinase activity and resulting in increased nuclear levels of PI5P. The accumulation of PI5P recruits ING2 to chromatin and promotes ING2-dependent p53 acetylation. Acetylation of p53 enhances its activity and stability and therefore increases apoptotic death. PI5P also may modulate an upstream activator of p38 MAPK, resulting in the activation of the Cul3-SPOP ubiquitin ligase complex toward multiple substrates, including PIPKIIβ. Represented are the defined functions of PI5P and PI4,5P2; however, both PI5P and PI4,5P2 may bind as of yet unidentified effectors (green boxes), which could play diverse roles in nuclear signaling. Ub = ubiquitin; A = acetylation