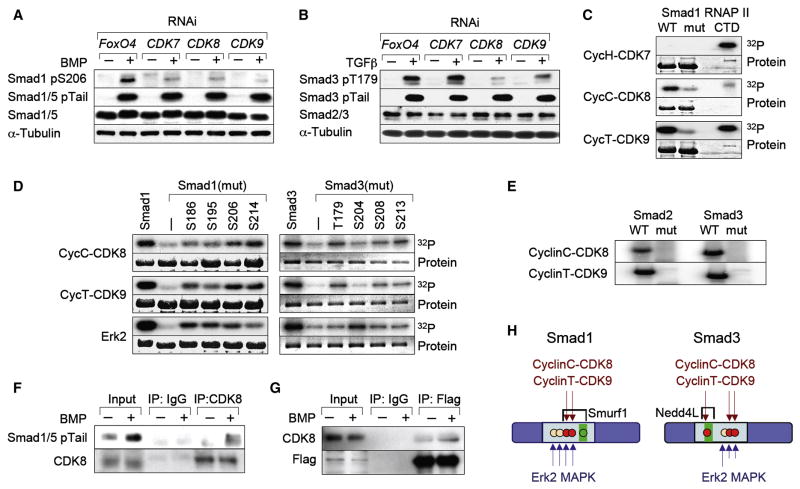
Figure 4. CDK8 and CDK9 as mediators of Smad ALP.
(A, B) HaCaT cells were transfected with siRNAs against CDK7, CDK8, CDK9 or against FoxO4 as a negative control. Total extracts from cells stimulated with BMP or TGFβ for 1 h were analyzed by immunoblot. (C) Autoradiograph of bacterially expressed Smad1 wild-type or linker mutant, phosphorylated in vitro by the indicated CDK/Cyclin complexes with γ32P-ATP as substrate. Purified CTD domain of RNA Pol II was used as a positive control for CDK activity. (D) In vitro kinase assay of the indicated CDK9/CyclinT, CDK8/CyclinC complexes or ERK, on bacterially expressed Smad1 and Smad3 wild-type or linker mutant. The numbers indicate the residue that was left as Ser/Thr in the linker region. (E) As in (C) but with Smad2 and Smad3 wild-type or linker mutants. (F) Co-immunoprecipitation of endogenous Smad1/5 and CDK8. Cell extracts from untreated or BMP-treated HaCaT cells were immunoprecipitated using an anti-CDK8 antibody and analyzed by western immunoblotting with antibodies against Smad1/5 pTail, or CDK8 as a loading control. (G) Flag immunoprecipitates of BMP-treated HaCaT cells stably expressing Flag-Smad1 were analyzed using antibodies against CDK8, or Flag as a loading control. (H) Schematic summary of CDK8/9 phosphorylation sites in Smad linker regions and their relationship to ubiquitin ligase recognition sites (Gao et al., 2009; Sapkota et al., 2007). Red dots, principal phosphorylation sites; green boxes, PY motifs.