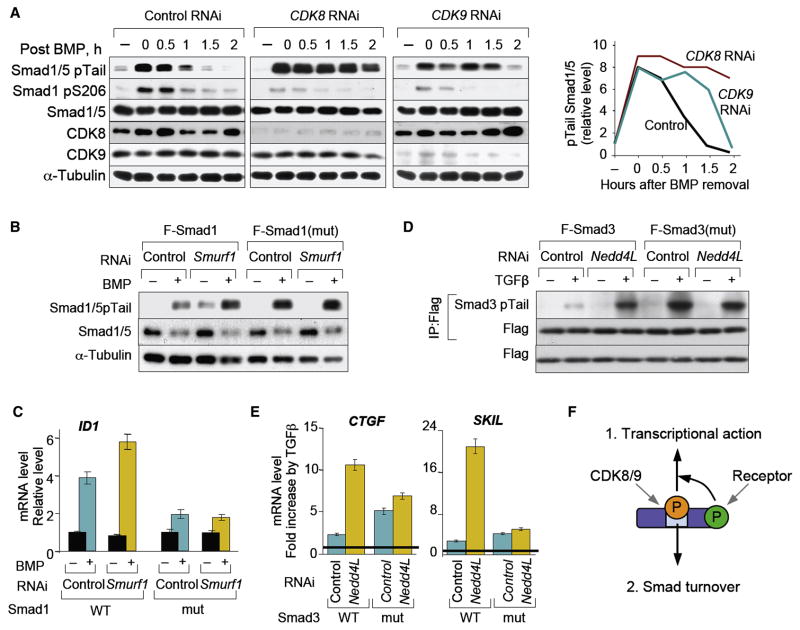
Figure 5. Role of CDK8/9 and linker phosphorylation in Smad turnover.
(A) Time course of Smad1/5 C-tail phosphorylation after 1 h of BMP stimulation as in Figure 2F. HaCaT cells transfected with siRNA against CDK8, CDK9 or the control FoxO4 were stimulated 48 h later cells and total lysates were analyzed by immunoblotting. The fourth panel shows a quantitation of the Smad1/5 pTail bands. (B) HaCaT cells with stable shRNA Smad1 knockdown and stably transduced with vectors encoding Smad1 wild-type or linker mutant were treated with BMP in the presence of Smurf1 or control siRNA and analyzed by immunoblotting. (C) The cell lines described in (B) were treated with BMP for 1 h and cells were harvested 2 h after BMP removal to test the expression of ID1 by qRT-PCR. Data show the mean ± S.D of quadruplicates and are representative of two independent experiments. (D) HeLa-S3 cells stably expressing Flag-tagged Smad3 (wild-type or linker mutant) were retrovirally infected with vectors encoding control shRNA or shRNA against Nedd4L. After treating cells with or without TGFβ for 3 h whole cell lysates were subjected to anti-Flag immunoprecipitation and analyzed by immunoblotting. (E) The cell lines used in (D) were treated with TGFβ for 3 h, and total RNA was isolated for qRT-PCR analysis of CTGF or SKIL levels. Values are normalized to the untreated levels, and shown as the fold induction by TGFβ in each cell line. Data are the mean ± S.D of quadruplicates and are representative of two independent experiments. (F) Schematic representation of the dual role of linker phopshorylation.