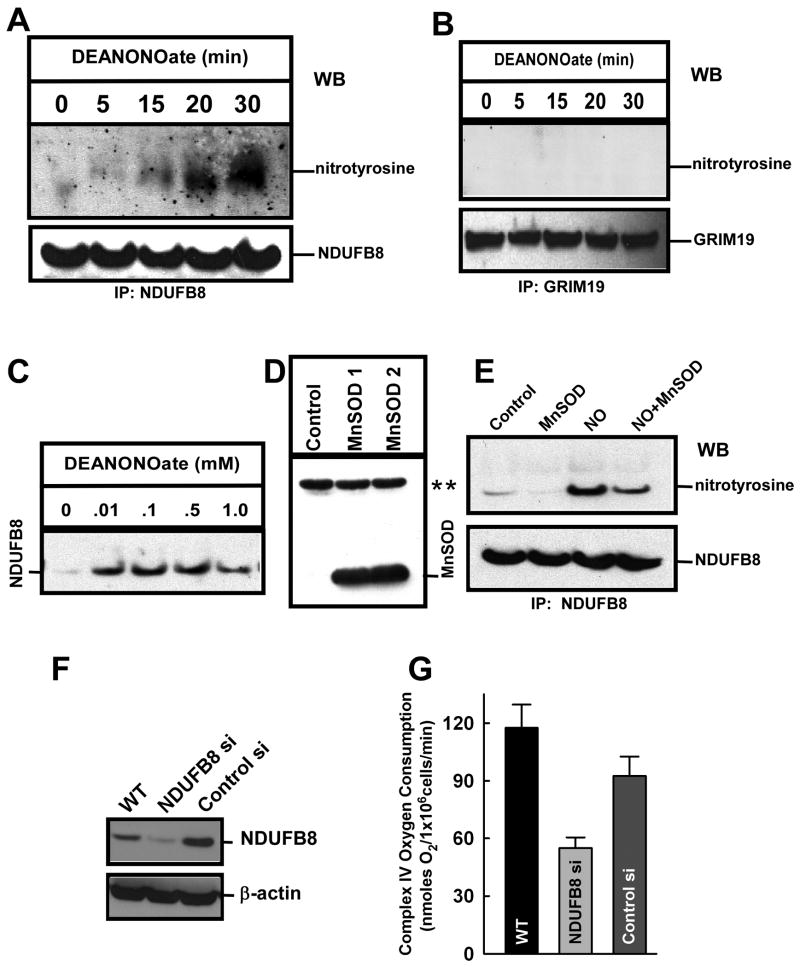
Figure 5.
Induction of NO-mediated complex I subunit NDUFB8 nitration in endothelial cells requires mitochondrial O2․-. (A) HPMVECs were permeabilized with ICM Buffer containing digitonin [40] and exposed to DeaNONOate (100 μM) for the indicated times. Immunoprecipitation was performed with NDUFB8 antibody and immunoblotted with an anti-nitrotyrosine antibody. Bound NDUFB8 protein was probed for specificity and equal loading. (B) Similarly, HPMVECs exposed to DeaNONOate (100 μM) for the indicated times were immunoprecipitated with 19 kDa Complex I (NADH dehydrogenase) subunit GRIM-19 and probed with anti-nitrotyrosine antibody. (C) Dose-dependent nitration of NDUFB8 by DeaNONOate. (D) HPMVECs were infected with Ad5CMVMnSOD and cell lysate analyzed by immunoblotting with anti-MnSOD antibody. ** indicates the nonspecific protein band as loading control. (E) HPMVECs overexpressing MnSOD and control cells were stimulated with 100 μM DeaNONOate for 30 min. Cell lysates were then immunoreacted with NDUFB8 antibody to detect NDUFB8 nitration. (F) Western immunoblotting demonstrated a significant reduction in NDUFB8 protein expression when HPMVECs were transfected with NDUFB8 siRNA as compared to control-siRNA transfected cells 48 hr post transfection. Representative immunoblot is shown of 2 independent measurements. (G) Mitochondrial oxygen consumption in response to the Complex IV substrates TMPD/Asc (0.4 mM/2.5 mM) was measured in digitonin-permeabilized HPMVECs (mean±S.E.M., n=3).