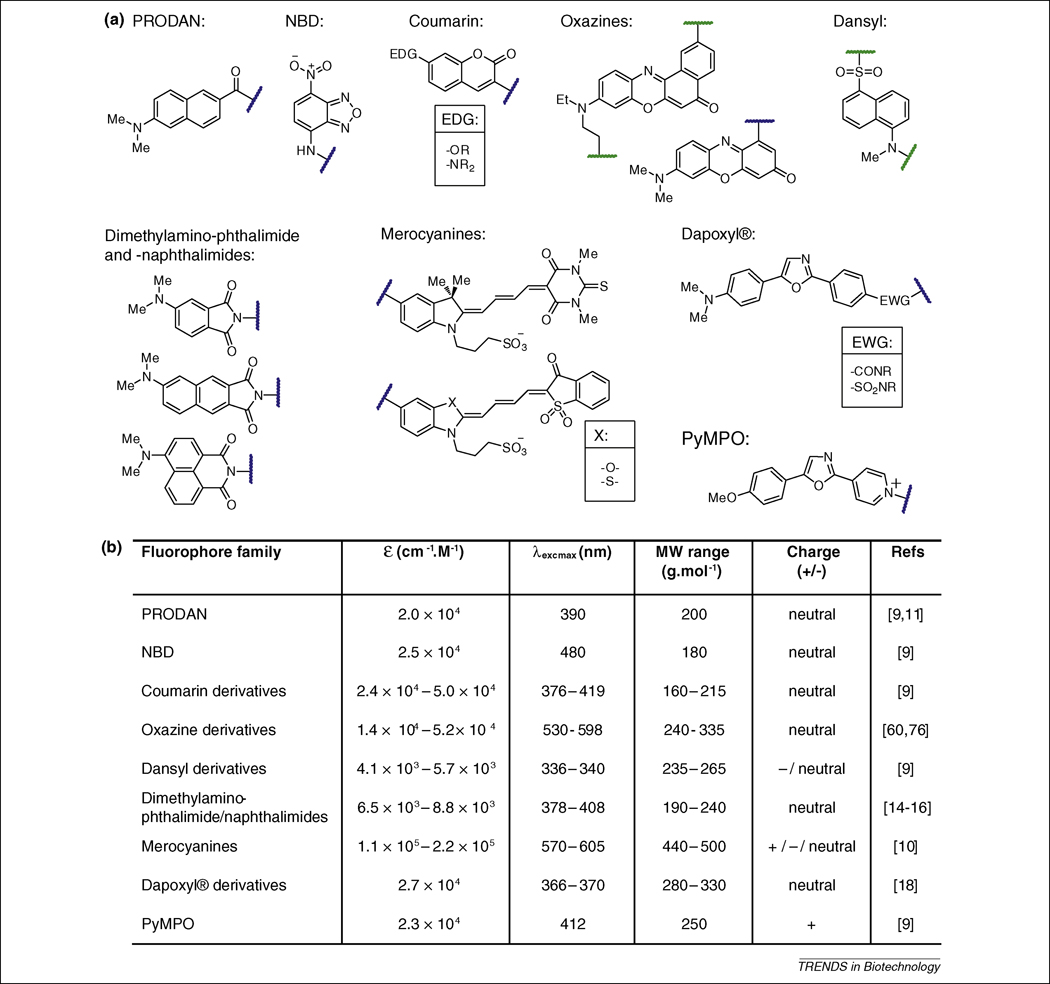
Figure 1. Established solvatochromic fluorophores commonly used in peptide and protein studies.
(a) Representative structures of the different families of solvatochromic fluorophores (PRODAN: 6-propionyl-2-(dimethylaminonaphthalene); NBD: 7-nitrobenz-2-oxa-1,3-diazol-4yl amine or nitrobenzoxadiazole; PyMPO: 1-(2-maleimidylethyl)-4-(5-(4-methoxyphenyl)oxazol-2-yl) pyridinium methanesulfonate). The position of attachment to biomolecules, either directly or via a reactive group, are indicated by wavy lines (blue when the position is unique and green when multiple alternative anchoring positions have been developed). Points of substituent variability within the structures are indicated by EDG (electron donating group), EWG (electron withdrawing group) and X (heteroatom). (b) Summary table of the solvatochromic fluorophores properties.