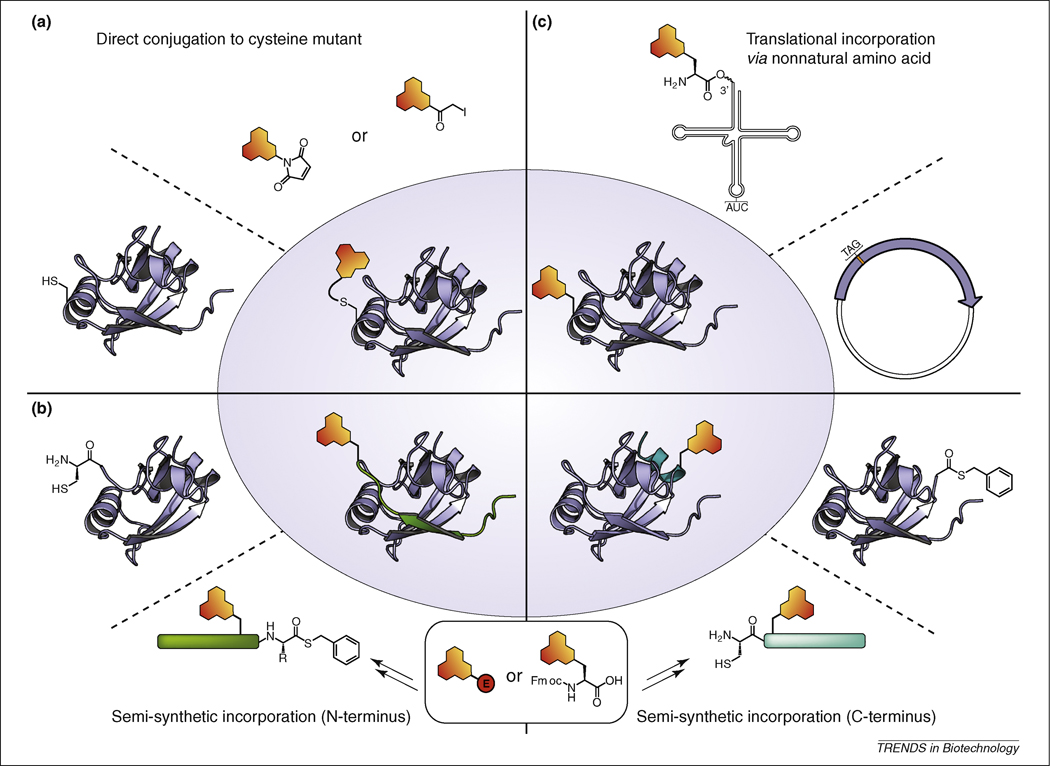
Figure 2. Methods for site-selective and site-specific incorporation of solvatochromic fluorophores into protein.
(a) Direct labeling of a solvent exposed cysteine residue using a thiol-reactive agent [9, 31]. (b) Incorporation of an unnatural amino acid possessing a solvatochromic fluorophore as the side chain group via suppression of the amber stop codon. A tRNA molecule designed to recognize and read-through the amber codon is charged with the unnatural amino acid [32, 42]. (c) Expressed protein ligation involves a semi-synthetic approach typically requiring either the N- or C-terminal end of the protein to be prepared by solid phase peptide synthesis (SPPS). The chromophore can be inserted either as an amino acid building block (e.g. Fmoc-protected amino acid) during SPPS, or afterwards by labeling a side-chain residue with an appropriate electrophilic derivative of the chromophore (represented as a red circle). The peptides are then ligated to the portion of the protein construct that was expressed from a recombinant gene product [33, 95, 96].