Figure 4. Design considerations.
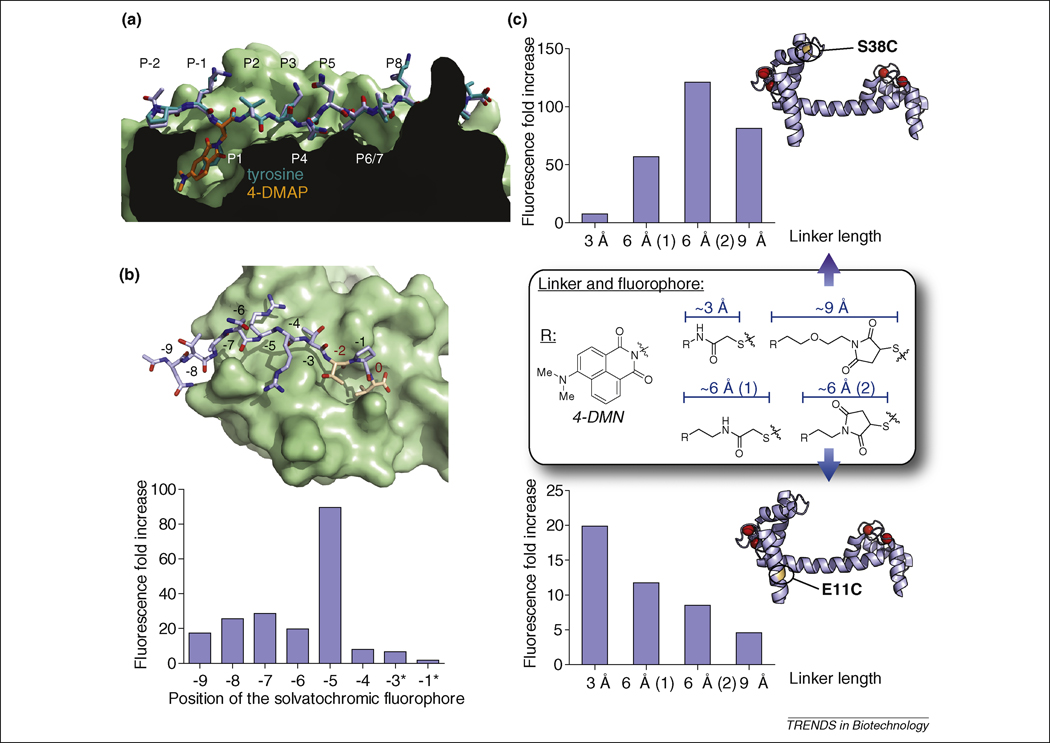
(a) Replacement of a conserved hydrophobic/aromatic residue by a solvatochromic fluorophore. Alignment of crystal structures of the (4-DAPA)-HA (2IPK, stick representation of the peptide in light blue with 4-DAPA highlighted in orange) and HA (1JWU, stick representation in teal) peptides bound to HLA-DR1 protein (class II MHC protein, surface representation), adapted from [17]. The replacement of the conserved ligand aromatic residue that occupies the P1 pocket of the HLA-DR protein by the 4-DAPA and 6-DMNA amino acids yielded highly efficient fluorogenic probes (over 1000-fold increase in fluorescence upon binding) without significantly affecting the specificity or affinity compared to the native interaction [17]. The crystal structures illustrate the ability of the small size 4-DAPA solvatochromic amino acid to replace the tyrosine of the native ligand. The fluorogenic probes have enabled the monitoring of in vivo regulation of cell-surface peptide-binding activity of class II MHC proteins in primary dendritic cells. (b) Screening for optimal positioning of the environment-sensitive fluorophore. Top: crystal structure of the third PDZ domain of PSD-95 with a modeled bound decapeptide ligand derived from the PDZ domain-binding motif of Stargazin in stick representation (NTANRRTTPV, adapted from 1TP3). Critical residues for PDZ domain-mediated interactions (at position 0 and -2) are represented in orange with red numbering. The 4-DMAP fluorophore was inserted systematically in each non-critical position with a diaminobutyric acid linker (*: except for -3 and -1, where a diaminopropionic acid linker was used instead). The respective fluorescence increases observed upon binding to the cognate PDZ domain are presented in the bar graph. The screening approach yielded a probe with a ~90-fold fluorescence increase by insertion of the fluorophore at position -5 [84]. (c) Screening for optimal linker length between the protein backbone and the environment-sensitive fluorophore 4-DMN. Cysteine labeling agent analogues derived from 4-DMN were incorporated into monocysteine mutants of calmodulin (illustrated with S38C and E11C mutants in the calcium-bound state, 1UP5) and compared at each position for the effect of the linker length on the ability of the solvatochromic fluorophore to report changes in its local protein environment upon binding of calcium [92].