Abstract
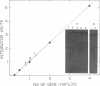
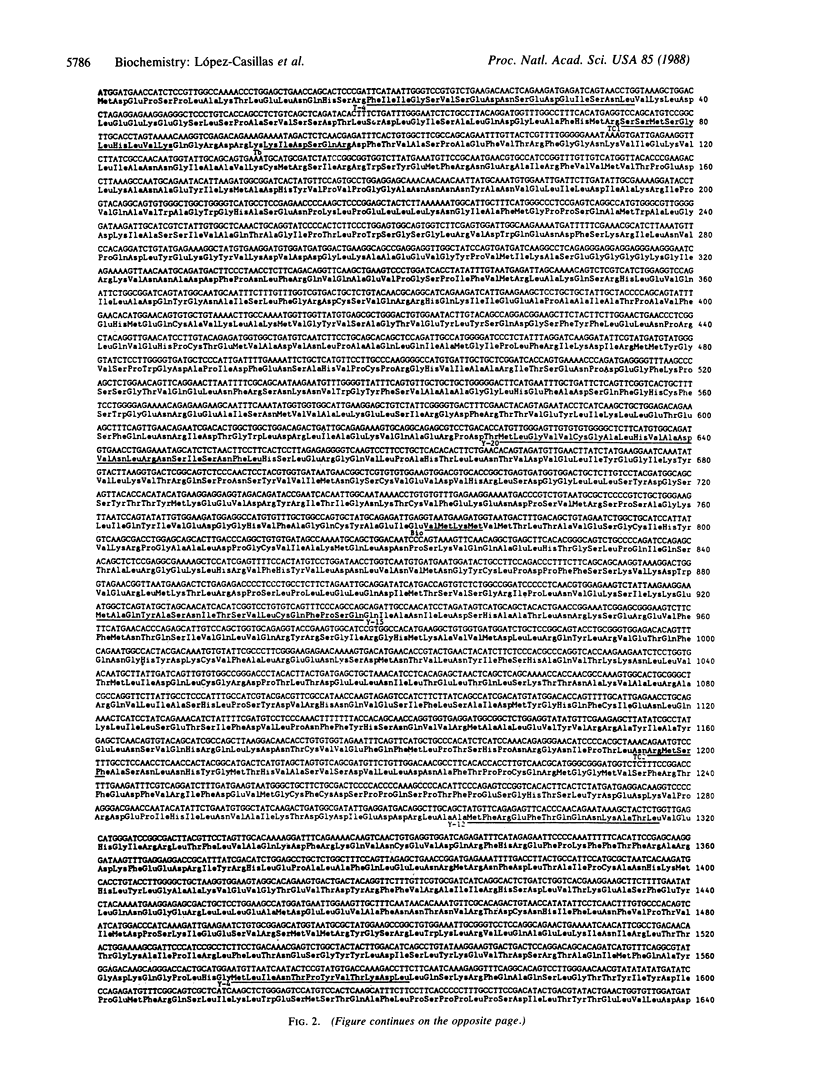
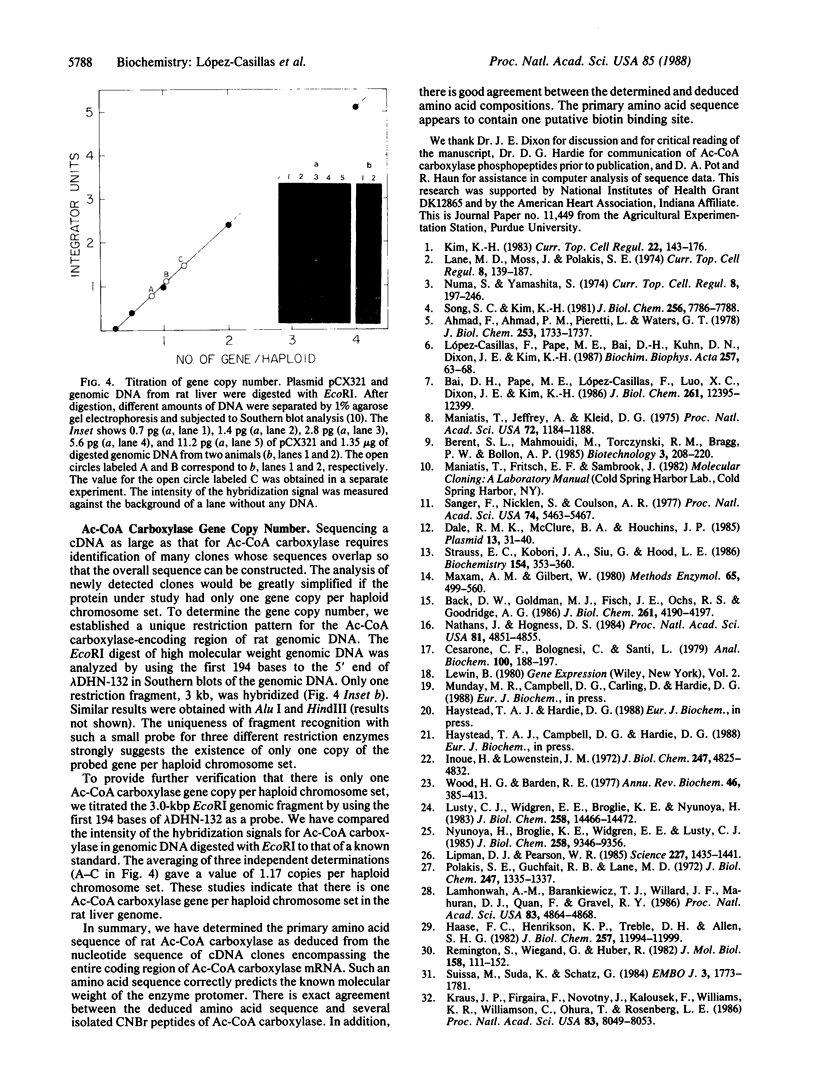
Acetyl-coenzyme A carboxylase (Ac-CoA carboxylase; EC 6.4.1.2) catalyzes the rate-limiting reaction in long-chain fatty acid biosynthesis. To investigate the mechanism of genetic control of expression of Ac-CoA carboxylase and the relationship between its structure and function, cDNA clones for Ac-CoA carboxylase were isolated. The complete coding sequence contains 7035 bases; it encodes a polypeptide chain of 2345 amino acids having a Mr of 265,220. The sequences of several CNBr peptides of Ac-CoA carboxylase were localized within the predicted protein sequence as were those peptides that contain the sites for phosphorylation. The deduced protein contains one putative site for biotinylation in the NH2-terminal half. The "conserved" biotinylation site peptide, Met-Lys-Met, is preceded by valine, whereas alanine is found in a similar position in all other known biotin-containing proteins. The primary sequences of Ac-CoA carboxylase and carbamoyl phosphate synthetase exhibit substantial identity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Ahmad P. M., Pieretti L., Watters G. T. Purification and subunit structure of rat mammary gland acetyl coenzyme A carboxylase. J Biol Chem. 1978 Mar 10;253(5):1733–1737. [PubMed] [Google Scholar]
- Back D. W., Goldman M. J., Fisch J. E., Ochs R. S., Goodridge A. G. The fatty acid synthase gene in avian liver. Two mRNAs are expressed and regulated in parallel by feeding, primarily at the level of transcription. J Biol Chem. 1986 Mar 25;261(9):4190–4197. [PubMed] [Google Scholar]
- Bai D. H., Pape M. E., López-Casillas F., Luo X. C., Dixon J. E., Kim K. H. Molecular cloning of cDNA for acetyl-coenzyme A carboxylase. J Biol Chem. 1986 Sep 15;261(26):12395–12399. [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Haase F. C., Henrikson K. P., Treble D. H., Allen S. H. The subunit structure and function of the propionyl coenzyme A carboxylase of Mycobacterium smegmatis. J Biol Chem. 1982 Oct 25;257(20):11994–11999. [PubMed] [Google Scholar]
- Inoue H., Lowenstein J. M. Acetyl coenzyme A carboxylase from rat liver. Purification and demonstration of different subunits. J Biol Chem. 1972 Aug 10;247(15):4825–4832. [PubMed] [Google Scholar]
- Kim K. H. Regulation of acetyl-CoA carboxylase. Curr Top Cell Regul. 1983;22:143–176. doi: 10.1016/b978-0-12-152822-5.50009-9. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Firgaira F., Novotný J., Kalousek F., Williams K. R., Williamson C., Ohura T., Rosenberg L. E. Coding sequence of the precursor of the beta subunit of rat propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8049–8053. doi: 10.1073/pnas.83.21.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamhonwah A. M., Barankiewicz T. J., Willard H. F., Mahuran D. J., Quan F., Gravel R. A. Isolation of cDNA clones coding for the alpha and beta chains of human propionyl-CoA carboxylase: chromosomal assignments and DNA polymorphisms associated with PCCA and PCCB genes. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4864–4868. doi: 10.1073/pnas.83.13.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M. D., Moss J., Polakis S. E. Acetyl coenzyme A carboxylase. Curr Top Cell Regul. 1974;8(0):139–195. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Widgren E. E., Broglie K. E., Nyunoya H. Yeast carbamyl phosphate synthetase. Structure of the yeast gene and homology to Escherichia coli carbamyl phosphate synthetase. J Biol Chem. 1983 Dec 10;258(23):14466–14477. [PubMed] [Google Scholar]
- López-Casillas F., Pape M. E., Bai D. H., Kuhn D. N., Dixon J. E., Kim K. H. Preparation of functional acetyl-CoA carboxylase mRNA from rat mammary gland. Arch Biochem Biophys. 1987 Aug 15;257(1):63–68. doi: 10.1016/0003-9861(87)90543-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa S., Yamashita S. Regulation of lipogenesis in animal tissues. Curr Top Cell Regul. 1974;8(0):197–246. doi: 10.1016/b978-0-12-152808-9.50012-2. [DOI] [PubMed] [Google Scholar]
- Nyunoya H., Broglie K. E., Widgren E. E., Lusty C. J. Characterization and derivation of the gene coding for mitochondrial carbamyl phosphate synthetase I of rat. J Biol Chem. 1985 Aug 5;260(16):9346–9356. [PubMed] [Google Scholar]
- Polakis S. E., Guchhait R. B., Lane M. D. On the possible involvement of a carbonyl phosphate intermediate in the adenosine triphosphate-dependent carboxylation of biotin. J Biol Chem. 1972 Feb 25;247(4):1335–1337. [PubMed] [Google Scholar]
- Remington S., Wiegand G., Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111–152. doi: 10.1016/0022-2836(82)90452-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. S., Kim K. H. Reevaluation of properties of acetyl-CoA carboxylase from rat liver. J Biol Chem. 1981 Aug 10;256(15):7786–7788. [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Suissa M., Suda K., Schatz G. Isolation of the nuclear yeast genes for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J. 1984 Aug;3(8):1773–1781. doi: 10.1002/j.1460-2075.1984.tb02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]