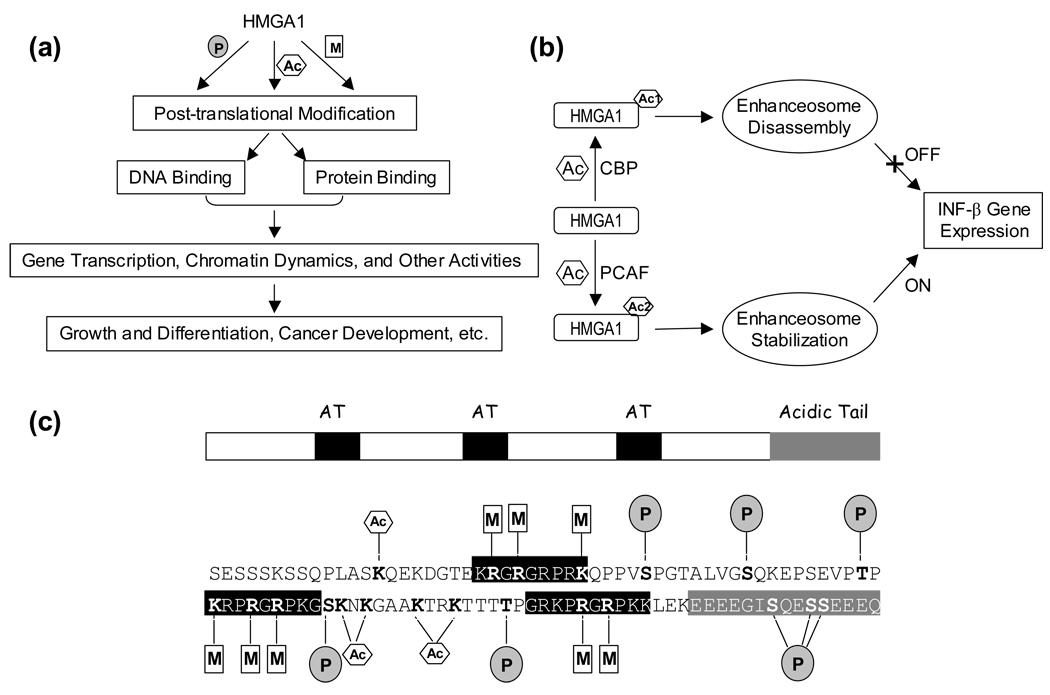
Figure 2.
(a) A schematic overview of the nuclear functions of post-translationally modified HMGA1 proteins. (b) An example showing how HMGA1 acetylation affects the expression of IFN-β gene [40, 41]. (c) Domain structure, primary sequence and the known major post-translational modifications of human HMGA1a with the modified residues being highlighted in bold. The three ‘AT-hook’ domains are shaded in black, and the acidic C-terminal tail is shaded in grey in the domain structure and primary sequence of the protein. Phosphorylation, methylation and acetylation are denoted by ‘P’ in shaded circle, ‘M’ in rectangle and ‘A’ in hexagon, respectively.