Figure 3. Interaction of Stat5a/b and androgen receptor (AR) signaling pathways in prostate cancer cells.
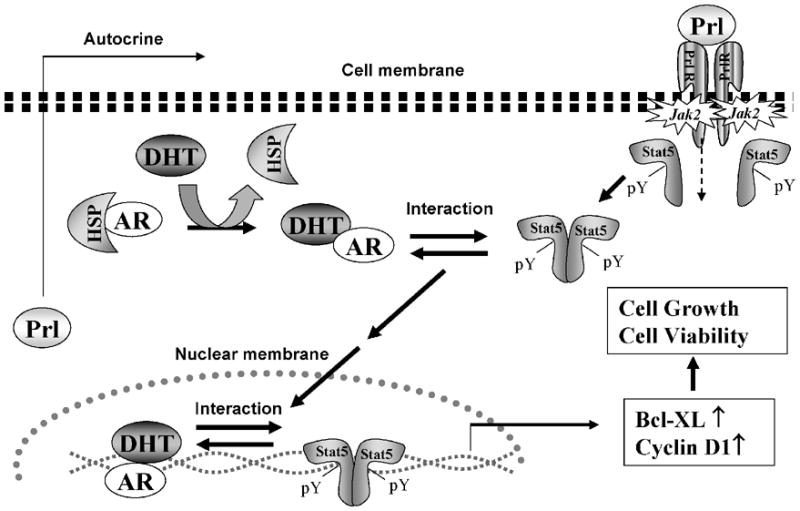
Binding of Prl leads to Prl-receptor (PrlR) dimerization and activation of Jak2 proteins pre-associated with the cytoplasmic domains of PrlR. Cytoplasmic Stat5a/b are recruited to the activated PrlR-Jak2 complex, Stat5a/b is phosphorylated on a conserved tyrosine residue in the C-terminus of Stat5a/b by Jak2 resulting in Stat5 dimerization. Ligand binding to the AR leads to its dissociation from the heat shock proteins (HSPs) and dimerization. Liganded AR physically interacts with Stat5a/b in prostate cancer cells. Liganded AR and active Stat5a/b promote nuclear translocation and transcriptional activity of each other. Both AR and Stat5 signaling pathways are critical regulators of growth, viability and apoptosis of prostate cancer cells.
