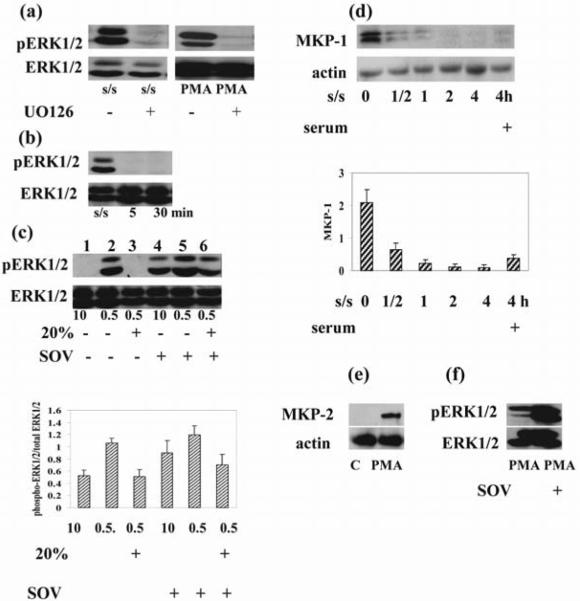
Figure 4. Pharmacological inhibition of MEK1/2 and phosphatases result in opposite outcomes.
(a) Thymocytes were cultured in medium in the presence or absence of UO126 (10 μM) for 1 hour and exposed to PMA (1 μM) for 30 minutes. Thymocytes were serum-starved for 6 hours in the presence or absence of UO126 (10 μM). (b) Addition of 20% serum to serum-starved (24 hours) cells suppressed ERK1/2 activation at 5 and 30 minutes. (c). Thymocytes were cultured in the presence or absence of sodium orthovanadate in medium with 0.5%, 10% serum and in medium with 0.5% stimulated with 20% serum. Addition of sodium orthovanadate to cells cultured in 10% serum as well as to serum starved cells after serum stimulation increased ERK1/2 activation. Densitometric quantification from (c) is shown. The phospho ERK1/2 bands were densitized and normalized to ERK1/2 immunoblotted from the same membrane to demonstrate the phosphorylation levels of ERKs. (d) Serum withdrawal suppressed, but serum addition activated MKP-1 as early as 30 minutes after its addition. Actin was used as a control for equal loading of protein. The histogram shows the MKP-1expression level relative to actin by densitometry. (e) PMA (1 μM) treatment induced MKP-2 activation. Actin was used as a control for equal loading of protein. (f) Thymocytes were cultured in medium in the presence or absence of sodium orthovanadate (100 μM) for 1 hour and exposed to PMA (1 μM) for 30 minutes. Sodium orthovanadate pretreatment increased ERK1/2 activation. Total and phosphorylated ERK1/2 as well as MKP-1, MKP-2 and actin was determined by immunoblotting. Representative blots from 3 experiments are shown in each (a)–(f).