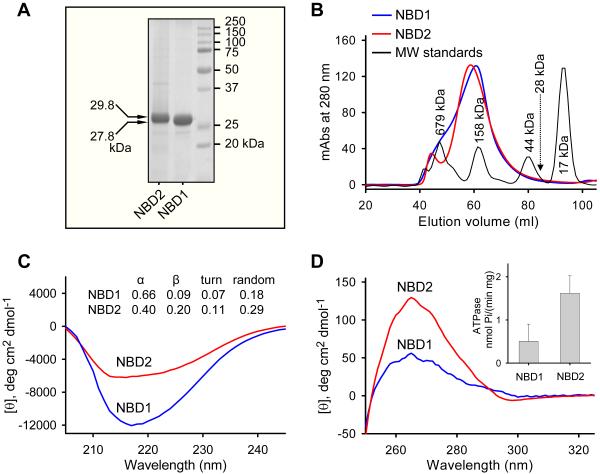
Figure 1.
Structural and functional integrity of refolded SUR2A NBD1/NBD2 proteins. Recombinant nucleotide binding domains, NBD1 and NBD2, were purified, refolded, and extracted from size exclusion chromatography. (A) Loaded at 7 μg/protein, Coomassie blue-stained NBD1 and NBD2 migrated at SDS-PAGE following expected molecular weights of 27.8 kDa and 29.8 kDa, respectively. (B) Superdex 200 gel filtration column elution of NBD1 and NBD2 demonstrated oligomeric profiles away from the monomeric 28 kDa protein. The far-UV (C) and near-UV (D) circular dichroism spectra revealed distinctive secondary and tertiary structures of NBD1 and NBD2. Inset: ATPase activity of NBD1 (0.51±0.37 nmol/mg/min, n=4) and NBD2 (1.62±0.41 nmol/mg/min, n=4) demonstrated functionality of refolded protein.