Abstract
A limited amount of functional recovery commonly occurs in the weeks and months after stroke, and a number of studies show that such recovery is associated with changes in the brain's functional organization. Measures that augment this reorganization in a safe and effective way may therefore help improve outcome in stroke patients. Here we review some of the evidence for functional and anatomical reorganization under normal physiological conditions, along with strategies that augment these processes and improve outcome after brain injury in animal models. These strategies include counteracting inhibitors of axon growth associated with myelin, activating neurons' intrinsic growth state, enhancing physiological activity, and behavioral therapy. These approaches represent a marked departure from the recent focus on neuroprotection and may provide a more effective way to improve outcome after stroke.
Stroke is the leading cause of serious disability among adults in the U.S. and many other countries. Over 700,000 people suffer a stroke each year in the U.S. Of these, 75–80% survive beyond the first year, and approximately half of the survivors are likely to sustain lifelong losses in sensory, motor, cognitive, behavioral, communicative, and/or emotional functioning, depending on the size and localization of injury. These numbers translate into a prevalence of some 4.4 million people in the U.S. with persistent disabilities due to stroke, and probably 20 times this number worldwide. With current figures on the costs of stroke care, long-term stroke disability costs an estimated $30 billion per year (Carmichael, 2006). In short, the impact of stroke is staggering in terms of loss of life, impact on productivity and quality of life for survivors and their families, loss of income, and medical expenses.
Effective treatment options are extremely limited. In the case of ischemic stroke, treatment with tissue plasminogen activator, a clot lytic, can be effective in limiting brain damage if applied within the first 4.5 hours (Del Zoppo et al., 2009), although this is often not feasible. An enormous effort has been directed towards reducing secondary injury after the initial ischemic or thrombotic event has occurred. However, although a great deal has been learned about the pathophysiological mechanisms that underlie secondary injury (Bramlett and Dietrich, 2004), this research has not yet been translated into gains for stroke victims (Dobkin, 2003; Ginsberg, 2008). At this point, post-stroke treatment remains limited to physical and occupational therapy.
An alternative approach to improving outcome might be to augment the brain's normal capacity to reorganize itself after injury. Studies in humans and laboratory animals show that areas of the brain that remain unaffected by injury can, to some extent, take over functions formerly mediated by the damaged areas. In some cases, these functional changes have been shown to correlate with changes at the dendritic and axonal levels that presumably enable areas interconnected to the damaged region to assume some of its functions. Although multiple factors are known to restrict anatomical reorganization in the adult CNS, a great deal of progress has been made in counteracting these factors, and this has led to marked improvements in animal models of stroke. These studies hold out the possibility that such methods may be used one day to improve functional outcome in stroke patients. Stem cell transplantation therapies may also stimulate axonal sprouting and other processes of anatomical reorganization in the brain adjacent to the stroke, and are discussed elsewhere (Bliss et al., 2010).
Functional reorganization after stroke
Most stroke patients show some degree of recovery after their initial stroke event. In many cases, limb function improves within the first month. Language and other cognitive domains also often show improvements during this period (Dobkin, 2003), with further improvements extending into the first year. What is the substrate for this recovery? Edema and the acute inflammatory response subside within the first several days of ischemic stroke, and this may produce some of the early recovery seen on the clinical stroke unit. Long-term improvements, on the other hand, are likely to be associated with changes in brain activity and organization.
The human brain responds to stroke with a reorganization of the anatomical representation of motor, sensory, language and other cognitive operations. This “remapping” of brain networks has been measured with functional imaging, direct cortical stimulation, and recently developed techniques to inactivate focal cortical areas with direct current or magnetic stimulation (Floel and Cohen, 2010; Wittenberg, 2010). Strokes acutely disrupt the co-activation of connected cortical networks in cognitive tasks (Grefkes et al., 2008; He et al., 2007). In the first weeks after stroke, cortical activation patterns during movement or language tasks reflect a widespread recruitment of areas ipsilateral and contralateral to the stroke site (Cramer, 2008; Dobkin, 2003; Ward and Frackowiak, 2006). With time and in a process correlated with recovery, this diffuse activation gives way to an activation of cortical areas that more closely resembles the non-stroke state: for example, primary and secondary motor and language areas ipsilateral to the stroke are activated in patients with good recovery in these modalities (Cramer, 2008; Ward and Frackowiak, 2006). Motor and sensory maps may also remap more locally in peri-infarct cortex immediately adjacent to a cortical stroke (Calautti et al., 2003; Cramer and Crafton, 2006). Cortical areas contralateral to the stroke also remain activated in motor, sensory and language tasks in many studies, and these contralesional areas may play a role in compensating for the loss of brain areas in the damaged hemisphere (Johansen-Berg et al., 2002; Winhuisen et al., 2005). Brain activation contralateral to the stroke is associated with larger strokes and poorer recovery (Cramer, 2008; Ward and Frackowiak, 2006), however, and the role of cortex contralateral to the stroke in recovery remains to be defined (Murase et al., 2004).
Cortical mapping studies in animals after stroke or focal brain lesions support the findings in humans. Using functional MRI, sensorimotor stimulation of the forelimb in the first days after focal stroke in the rat activates contralesional cortex to a greater degree than in normal rats. After two weeks, the peri-infarct cortex becomes more responsive to sensorimotor stimulation, and this shift correlates with behavioral recovery (Dijkhuizen et al., 2003). More precise optical mapping of sensorimotor responses, and with smaller strokes confined to forelimb sensorimotor cortex, shows a very early shift of cortical sensorimotor responses to areas in peri-infarct cortex (Sigler et al., 2009), and a later remapping of forelimb responses within other regions of peri-infarct cortex, including hindlimb areas (Brown et al., 2009; Winship and Murphy, 2008). Small lesions in the forelimb motor cortex of the rat, combined with precise mapping of motor cortical areas, indicates that recovery of function associated with neurorehabilitative training involves a recruitment of premotor cortex into low threshold motor activation (Conner et al., 2005). These data indicate that experimental stroke induces a pattern of distributed brain activity in motor and sensory maps, followed by a reorganization of cortical activity into adjacent regions of peri-infarct cortex.
Dendritic changes after stroke
Anatomical studies show that dendritic spines undergo remodeling in peri-infarct cortex. Within hours of a stroke, many neurons destined to survive the infarct lose their dendritic spines (Brown et al., 2008). Increases in dendritic spine number and in spine turnover rates occur maximally within the first two weeks after stroke in cortex that is within millimeters of the infarct (Brown et al., 2009). When observed in real time with two-photon imaging, these studies demonstrate a period of rapid synaptogenesis and circuit plasticity within the first weeks after stroke. These changes in dendritic spine structure, and presumably synaptic innervation, occur within limb somatosensory areas that exhibit remapping after stroke (Winship and Murphy, 2008). Changes in dendritic branching are also seen in the ipsilateral parietal cortex after unilateral, non-stroke, frontal injury (Kolb and Gibb, 1991). Other studies demonstrate structural changes at the synaptic level in the opposite hemisphere if animals come to rely on the unimpaired limb. Unilateral lesions of the forelimb motor area result in increased dendritic branching in layer 5 neurons of the undamaged, contralateral forelimb motor area (Jones et al., 1996) and increased numbers of dendritic spines per neuron (Jones and Schallert, 1994). These changes are use-dependent: they are blocked if rats are prevented from using the unimpaired limb, and are amplified if animals are able to use that limb to perform complex tasks (Jones et al., 1999; Jones and Schallert, 1994). Exposing rats to an enriched environment also increases dendritic spine complexity in cortex contralateral to a stroke site (Biernaskie and Corbett, 2001). Dendritic spine turnover is enhanced with blockade of the neuronal growth inhibitor NogoA (Papadopoulos et al., 2006). Although the dendritic reorganization described in these studies is likely to contribute to functional recovery, causal relationships have not, in general, been proven as yet.
Axonal reorganization after CNS injury
The ability of CNS neurons to extend axon branches and form new connections after injury has been known for over 35 years. Transecting the fimbria on one side of the brain eliminates synaptic connections from one hippocampus to the ipsilateral septum. Within a month or so, axons arising from the contralateral hippocampus sprout terminal branches and form new synapses that replace nearly all the ones that had been lost (Raisman and Field, 1973). Within the hippocampal formation, dendrites of dentate granule cells receive inputs from several sources, including the ipsilateral entorhinal cortex and the contralateral hippocampus. Lesions of the perforant pathway eliminate entorhinal inputs that terminate on the apical dendrites of dentate granule cells. Over a relatively short period of time, projections from the contralateral hippocampus expand and form new connections that replace synapses which had been lost (Lynch et al., 1973).
Axonal sprouting also occurs in experimental stroke models. As noted above, dendritic spine rearrangement occurs in peri-infarct cortex and contralateral cortex after stroke. Because cortical spines have experimentally always been found as postsynaptic partners with axonal boutons, one would expect a priori that axonal sprouting is also taking place, and indeed this has been demonstrated by quantitative analysis of cortical projection patterns using axonal tracers. Because of the widespread and dense connections of cortical areas ipsilateral to any given part of cortex, such an approach requires careful mapping of cortical projections. In the rat barrel cortex, the stereotyped nature of the connections has allowed determination of a post-stroke axonal sprouting response that is robust and remaps the normal body representation in this somatosensory area (Carmichael et al., 2001). Longer distance axonal sprouting also occurs in cortical connections within the hemisphere ipsilateral to the stroke site. Small photothrombotic strokes in forelimb sensorimotor cortex produce new patterns of cortical connections in adjacent areas of retrosplenial cortex (Brown et al., 2009).
Massive axonal rearrangements are likewise seen in primates after brain injury (Dancause et al., 2005). The primary motor area is reciprocally connected to premotor and somatosensory areas via circuits that integrate movement with tactocutaneous and somatosensory activity. Lesions of the primary motor area eliminate major inputs to some of these other areas. Over time, new connections develop from the premotor area to the primary sensory cortex, replacing inputs from the primary motor area that had been lost. Although these studies focused on one particular pathway, there is no reason to believe that it is the only one that undergoes significant reorganization. Nudo has stated that “Regardless of the functional significance of this finding, it is now clear that we can no longer consider the injured brain as a normally wired brain with a missing puzzle piece” (Nudo, 2006). Presumably, changes like the ones found here underlie the shift in functional maps described above by Conner et al. and other groups.
Long descending pathways that originate in the sensorimotor cortex also become reorganized after injury. The corticospinal tract (CST) projects in a topographically organized fashion from sensorimotor areas in the cortex to the spinal cord, and mediates voluntary movements of the distal musculature. Damage to this pathway in rats causes a complete loss of skilled reaching with the forepaws (Weidner et al., 2001) and other skills that require sensorimotor integration. Damage to either the dorsal CST, which carries about 95% of all descending axons, or the ventral CST, which carries only about 2% of the axons, results in a loss of skilled food retrieval which recovers with time. In the case of dorsal CST lesions, functional recovery coincides with the sprouting of collateral branches from ventral CST axons that form new synapses on motor neurons. Subsequent lesions of the ventral CST result in complete loss of fine reaching (Weidner et al., 2001). Similarly, the recovery that occurs after lesioning the ventral CST is presumably due to sprouting of spared dorsal CST axons, but this is technically difficult to demonstrate because of the numerous dorsal CST fibers that are already present. In addition to the ipsilateral sprouting described above, considerable CST sprouting occurs from one side of the spinal cord to the other. Following unilateral stroke or surgical transection of one CST, axons from the undamaged CST sprout collaterals that cross over to the side of the spinal cord that has lost its normal innervation (Lee et al., 2004; Zai et al., 2009).
Together, these studies show that stroke and other kinds of brain injury lead to the formation of new local circuits (Carmichael et al., 2001), long-distance intracortical connections (Brown et al., 2009; Dancause et al., 2005) and long, descending projections to the spinal cord (Lee et al., 2004; Weidner et al., 2001; Zai et al., 2009) (Figure 1). Nonetheless, the extent of this reorganization remains constrained by the many growth-inhibitory molecules that are present in neurons' extracellular environment, the limited availability of appropriate trophic agents, and factors that are intrinsic to neurons themselves. As described below, strategies that address these issues can enhance rewiring and dramatically improve functional outcome in animal models of stroke or of other types of CNS injury.
Figure 1. Patterns of axonal sprouting after stroke.
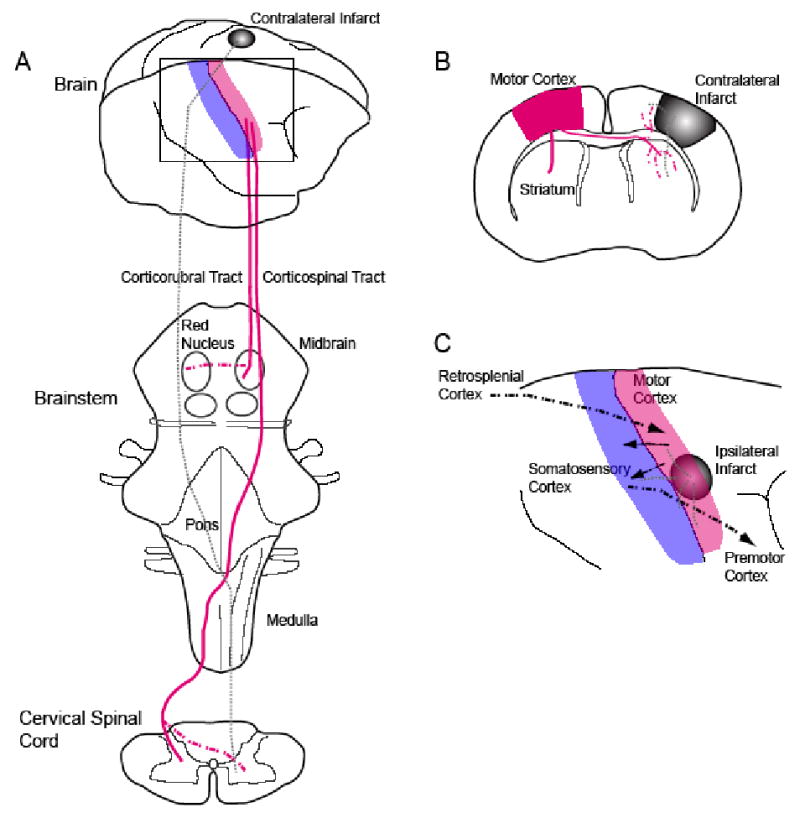
(A) Schematic view of motor cortex (red) projections from the brain to the brainstem and cervical spinal cord. In each panel the normal axonal projections from motor cortex are in solid lines. The projections from the cortical region lost from the stroke are in gray dashed lines. The projections that form after stroke are in red dashed lines. In (A), a stroke induces axonal sprouting from contralateral motor cortex into the red nucleus (Papadopoulos et al., 2002) or into the ipsilateral cervical spinal cord (Chen et al., 2002; Zai et al., 2009). These projections form in areas that were denervated by the loss of the stroked cortex. In (B), a stroke induces axonal sprouting from contralateral motor cortex into the ipsilateral striatum and contralateral peri-infarct cortex (Carmichael and Chesselet, 2002). These are areas in which the projections from the stroke site are lost. Stroke also induces several patterns of axonal sprouting in cortex adjacent or ipsilateral to the stroke site, shown in (C). New projections from retrosplenial cortex to peri-infarct cortex form after small strokes in motor cortex (Brown et al., 2009). Somatosensory cortex establishes new direct projections to the ventral premotor cortex. This pattern of axonal sprouting, identified in the squirrel monkey, establishes a long-distance projection from parietal to frontal lobe (Dancause et al., 2005). Premotor cortex in the rate is a site of motor remapping that correlates with functional recovery after cortical injury (Conner et al., '05). Stroke also causes a substantial axonal sprouting response in peri-infarct cortex near the stroke site (Carmichael et al., 2001).
Enhancing recovery by counteracting inhibitory signals
A number of molecules associated with CNS myelin, the perineuronal net, and the scar that forms at an injury site inhibit axon growth. Three proteins associated with myelin, NogoA, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin-associated protein (Omgp), exert their effects in part via a shared receptor complex comprised of the Nogo receptor (NgR), Lingo, and P75NTR or TROY (Filbin, 2003) and, at least in culture, via the PirB receptor (Atwal et al., 2008). MAG and an N-terminal region of NogoA can additionally inhibit axon growth through an integrin-related mechanism (Goh et al., 2008; Hu and Strittmatter, 2008) and MAG can act also act via NgR2 and gangliosides (Fujitani et al., 2005; McKerracher, 2002; Schnaar et al., 1998; Venkatesh et al., 2005). In addition to these 3 inhibitory proteins, myelin contains axon-repellants of the Semaphorin and ephrin families (Benson et al., 2005; Goldberg et al., 2004). The perineuronal net and the scar that forms at an injury site contain chondroitin- and keratin-sulfate proteoglycans (Silver and Miller, 2004) together with other molecules that are inhibitory to growth (Liu et al., 2006). Because axons within the mature CNS face such an array of inhibitory molecules, strategies that counteract only one or a few of these at a time might be expected to have only limited effects after stroke. In some cases, however, these effects can be dramatic.
One approach to counteracting inhibition has been to use antibodies directed against the myelin protein, NogoA. One study used a cell-based approach to deliver an anti-Nogo antibody (IN-1) into the cerebral ventricles of rats that had sustained a unilateral stroke in the distribution of the middle cerebral artery (MCA). These strokes disrupt, among other pathways, cortical efferents to the ipsilateral red nucleus (via the corticorubral tract) and to the contralateral spinal cord (via the CST). IN-1 treatment enhanced the growth of collateral branches from intact corticorubral axons that arise in the intact hemisphere into the denervated red nucleus and improved fine- motor control using the paw contralateral to the damaged hemisphere (Papadopoulos et al., 2002). IN-1 also promotes dendritic changes in layer 5 pyramidal cells contralateral to the side of the brain that sustained a stroke (Papadopoulos et al., 2006). It is important to remember that the anatomical changes reported in these and similar studies may represent the tip of the iceberg, and that more subtle anatomical changes that may also be occurring may also contribute to the observed behavioral improvements.
The effects of counteracting myelin-associated inhibitors after stroke have also been studied by deleting the genes that encode Nogo or NgR, or by using an NgR antagonist (Lee et al., 2004). As mentioned above, following a unilateral stroke, neurons in the undamaged hemisphere show some ability to extend collateral branches into areas of the brainstem and spinal cord which have lost their normal innervation. Deletion of the genes encoding Nogo or NgR increases this sprouting several-fold and improves skilled use of the impaired paw. An alternative way to block signaling through NgR is by using a soluble, recombinant form of the Nogo receptor that acts as a decoy and prevents the cognate ligands on myelin from binding to the receptors on growth cones. Like Nogo- or NgR-null mice, rats treated with the soluble decoy receptor show increased sprouting of CST and rubrospinal projections after strokes in the territory of the MCA, along with large improvements in their ability to use the impaired forelimb (Lee et al., 2004). Deletion of the Nogo receptor enhances the potential for anatomical reorganization within the cortex even in the absence of injury. In the primary visual cortex, inputs arising from the two eyes are normally sorted into separate zones within each hemisphere during a “critical period” of development. Mice lacking the gene for NgR retain the ability to change this pattern of connections even as adults (McGee et al., 2005). Thus, agents that block signaling through the Nogo receptor enhance plasticity at the axonal, dendritic, and synaptic levels and improve outcome after stroke.
As noted above, proteins associated with the perineuronal net and the scar that forms around an injury site are also highly inhibitory to axon growth. Some of the molecules that mediate this inhibition are chondroitin sulfate and keratin sulfate proteoglycans (CSPGs, KSPGs). The enzyme chondroitinase ABC removes critical inhibitory side-chains from these molecules and promotes axon growth after injury (Bradbury et al., 2002; Galtrey et al., 2007) and synaptic plasticity in the cortex (Pizzorusso et al., 2002). Following stroke in lab animals, CSPG levels decrease in the peri-infarct area, and this may contribute to the anatomical plasticity observed in such regions (Carmichael et al., 2005). Whether agents that further reduce CSPG levels would be beneficial after stroke has not been reported as yet.
Enhancing recovery by reactivating neurons' growth state
An alternative way to promote rewiring after stroke is through the use of agents that activate neurons' intrinsic growth potential. CNS neurons extend axons readily during development but generally lose this capacity at some point in the perinatal period (Goldberg et al., 2002). This transition is associated with a decline in the expression of a constellation of proteins that includes, among others, the membrane phosphoprotein GAP-43, certain cytoskeletal proteins, and particular adhesion molecules. The expression of these growth-associated proteins is reactivated in the peripheral nervous system (PNS) after injury but not, in general, within the CNS. However, this situation can change if neurons are presented with a more favorable extracellular environment or are stimulated with appropriate trophic agents (Benowitz and Routtenberg, 1997; Fischer et al., 2004; Skene, 1989). Gene expression analysis of peri-infarct cortex during the period of dendritic and axonal sprouting suggests that a neuronal “growth-associated program” becomes reactivated, although the pattern or gene changes seen after brain injury in mature animals differs substantially from that associated with PNS regeneration (Carmichael et al., 2005). This gene expression profile differs even more in the aged brain after stroke (Li and Carmichael, 2006), presumably reflecting an altered regenerative response that may be more representative of what happens in the aged human population in which stroke most commonly occurs.
GAP-43 expression increases during axon sprouting (Benowitz et al., 1990), and several studies have used GAP-43 immunostaining as a surrogate measure of axon growth and/or terminal sprouting in animal models of stroke. Following a stroke in rats that includes the parietal (sensory) cortex, levels of GAP-43 immunostaining increase markedly in peri-infarct areas of the ipsilateral cortex (Stroemer et al., 1995). These increases are further augmented with D-amphetamine, an agent that enhances functional recovery (Stroemer et al., 1998). Other studies have shown that the trophic factor FGF2 augments GAP-43 immunostaining in the sensorimotor cortex contralateral to the injured side of the brain (Kawamata et al., 1997), and that blockade of GAP-43 expression using antisense oligonucleotides suppresses the functional improvements seen with FGF2 treatment (Kawamata et al., 1999). Other polypeptide growth factors have been shown to promote axon regeneration or collateral sprouting in different injury models (Grill et al., 1997; Lu et al., 2004; Ramer et al., 2000; Schnell et al., 1994; Vavrek et al., 2006; Yin et al., 2006), though their effects after stroke has not been reported.
Axon growth is affected by intracellular levels of the second messenger, cAMP. cAMP activates gene expression programs that render axons unresponsive to inhibitory signals (Filbin, 2003), induce the translocation of growth factor receptors to the cell surface (Meyer-Franke et al., 1998), and alter the response of growth cones to axon repellants (Song et al., 1998). In vivo, elevation of cAMP stimulates regeneration of sensory axons after spinal cord injury (Neumann et al., 2002) and augments the growth-promoting effects of other polypeptide trophic factors (Lu et al., 2004). One study has reported that phosphodiesterase inhibitors, drugs that suppress the degradation of cAMP, increase the remapping of motor control and improve use of an impaired limb after unilateral strokes in the forelimb motor area (MacDonald et al., 2007). Other agents that would also be of interest to investigate are inhibitors of histone deacetylases (HDACs). HDAC inhibitors prevent the deacetylation of histone proteins, thereby favoring gene transcription. Certain HDAC inhibitors also affect cytoskeletal organization and other cellular functions. HDAC inhibitors are neuroprotective in animal models of stroke and promote synaptic plasticity in the mature brain (Langley et al., 2009). Whether they augment axon growth after stroke is as yet unknown.
One molecule that enhances axon growth and improves outcome in a rat stroke model is inosine. Inosine is a naturally occurring purine nucleoside that gets transported across the cell membrane and activates Mst3b, a protein kinase that regulates the cell-signaling pathway through which trophic factors induce axon growth (Irwin et al., 2006; Lorber et al., 2009). Additional benefits of inosine after brain injury include its anti-inflammatory effects (Hasko et al., 2004), its ability to suppress glutamate-induced neural excitation (Shen et al., 2005), and the ability of its metabolite, uric acid, to protect proteins from peroxynitrite-induced damage (Scott et al., 2005, 2002). Following unilateral cortical damage, inosine enhances the ability of undamaged neurons to extend axon collaterals into areas that have lost their normal innervation; this growth is accompanied by improved performance with the limb contralateral to the stroke (Chen et al., 2002; Zai et al., 2009). As illustrated in Fig. 2, sustained delivery of inosine after injury to the caudal forelimb area in one hemisphere increases the ability of pyramidal cells on the undamaged side of the brain to extend axon collaterals and form synapses on the denervated side of the spinal cord (Fig. 2a-c). After 4 weeks, animals treated with inosine are far superior to saline-treated animals in their ability to use the paw contralateral to the damaged hemisphere in a test requiring fine motor control (Fig. 2d: Zai et al., 2009). Similar results have been obtained in studies using a closed-head injury model (Smith et al., 2007). At the molecular level, stroke on one side of the brain causes layer 5 pyramidal cells in the opposite hemisphere to undergo many changes in gene expression, presumably related to denervation and stress. Inosine reverses many of these changes, while at the same time inducing the expression of genes related to axon growth and the complement cascade (Zai et al., 2009). The latter changes may be of particular significance in view of recent studies linking the complement cascade to synaptic reorganization (Stevens et al., 2007).
Figure 2. Inosine enhances axonal rewiring and augments functional recovery in an animal model of stroke.
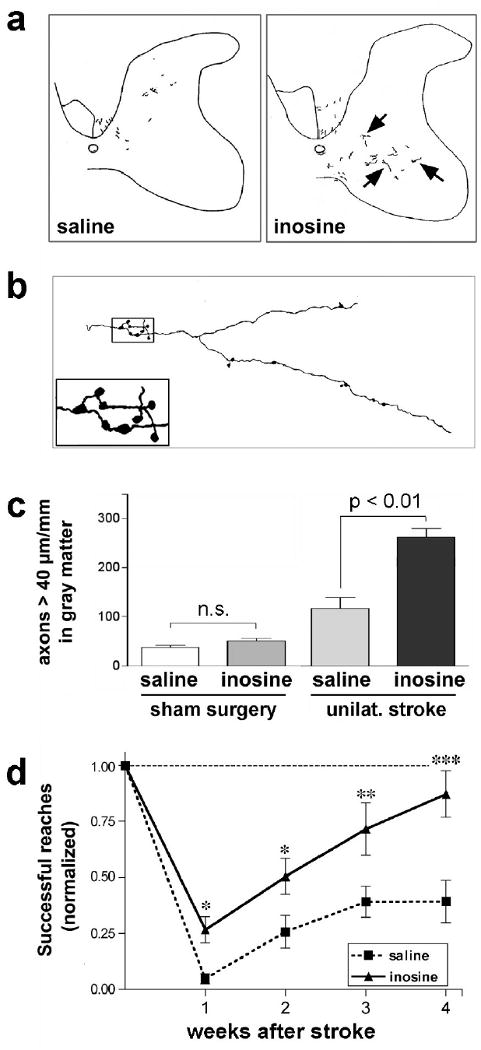
a Camera lucida drawings of BDA-labeled corticospinal tract (CST) fibers that originate in the uninjured hemisphere and project to the side of the spinal cord denervated by injury to the contralateral sensorimoter cortex. Rats were treated with either saline- (left) or inosine (right). b Higher-magnification camera lucida tracing of a fiber in a. Inset shows the presence of synaptic bouton-like structures. c Quantitation of ipsilaterally projecting CST fibers ≥ 40 μm in length in the transverse plane in the denervated dorsal funiculus and gray matter, respectively. Results are normalized by the intensity of staining in the intact CST and are reported as the number of labeled axons per mm length of spinal cord. Inosine does not promote CST axon growth in the absence of brain injury, but amplifies the amount of axon sprouting after a stroke. d Inosine enhances functional recovery. Animals were trained to retrieve food pellets through a restricted opening with either paw prior to surgery and were then tested beginning one week later by a blinded observer. Scores are reported as percentage of pre-operative performance. *, **, ***: Differences significant at P < 0.05, P < 0.01, P < 0.001, respectively. Error bars represent SEM. (from Zai et al., 2009).
Because activation of neurons' intrinsic growth state and counteracting cell-extrinsic inhibitors of growth involve different mechanisms, one might expect that the combining the two could have synergistic effects. This notion is borne out in studies of CNS axon regeneration: combining a stimulus that puts neurons of the retina in an active growth state (intraocular inflammation) with a treatment that renders growth cones “blind” to inhibitory signals in the extracellular environment (expression of an enzyme that inactivates the GTPase RhoA) results in far greater regeneration than either treatment alone (Fischer et al., 2004). Analogous combinatorial treatments also appear be effective after stroke. Combining inosine with a peptide that prevents Nogo from binding to NgR results in greater anatomical reorganization and functional recovery after stroke than either treatment alone (L. Zai, L. Havton, N. Irwin, S. Strittmatter, and L. Benowitz, in preparation).
Studies using a different injury model in rats, partial transection of the spinal cord, show that inosine is as effective when delivered intravenously as when infused intraventricularly (L. Zai et al., in preparation). In addition, inosine has a history of human use in cardiac patients (Czarnecki and Czarnecki, 1989), athletes (Starling et al., 1996), and in clinical trials for multiple sclerosis (http://clinicaltrials.gov/ct2/show/NCT00067327) and Parkinson's Disease (http://clinicaltrials.gov/ct2/show/NCT00833690). In view of its beneficial effects in animal models of stroke, proven safety in humans, and bioavailability, inosine might be considered for clinical trials after stroke. However, further preclinical work will be needed to define the appropriate dosage for i.v. delivery, the therapeutic window, and the generality of inosine's effects in other species and stroke models. It will also be important to establish whether inosine augments changes in brain organization beyond those seen in the CST, e.g., in intracortical associations.
Physiological activity promotes axon growth and synaptogenesis
During development, the pattern of synaptic connections is refined by activity-dependent competition among inputs. This phenomenon has been best characterized in the visual system (Katz and Shatz, 1996), but it also occurs elsewhere during development, including in the CST projection from sensorimotor cortex to the contralateral spinal cord (Martin, 2005). Surprisingly, persistent unilateral activation of the CST promotes axonal reorganization and synaptogenesis in mature rats even without injury. As noted above, unilateral CST injury stimulates axon sprouting from intact ipsi- and contralateral fibers, and this sprouting becomes even stronger when unilateral CST injury is combined with electrical stimulation of the intact CST. This combination results in a marked increase in the number and length of axon collaterals that cross from the normally innervated side of the spinal cord into the denervated side compared to either treatment alone, and is accompanied by a marked strengthening of functional connectivity between the intact side of the brain and the ipsilateral musculature (Brus-Ramer et al., 2007). Whether global activation of the intact CST results in the formation of connections that are behaviorally useful has not been reported. Studies in both patients and animals demonstrate improved use of an impaired limb following transcranial magnetic stimulation of undamaged pre-motor areas, although a recent Phase III clinical trial failed to show a significant benefit (Yozbatiran et al., 2009).
Activation under more physiological conditions strongly improves use of a disabled fore-limb after unilateral stroke or CST injury. This phenomenon is illustrated in the “forced use” paradigm. Following unilateral stroke, constraint of the unimpaired limb forces human patients or experimental animals to use the impaired forelimb, and this results in improved performance with that limb (Wolf et al., 2006). In rats with a unilateral transection of the CST, forced use of an impaired limb doubles the number of collateral branches that extend from the intact CST into the denervated side of the spinal cord. This growth is accompanied by the formation of new synapses and improved motor performance (Maier et al., 2008).
These studies highlight an important aspect of the studies of axonal sprouting and the field of neural repair after stroke: pharmacological or cellular therapies that promote recovery are acting within a brain that is exposed to altered behavioral activity patterns. These altered activity patterns may promote recovery and promote axonal sprouting in cortical circuits that are associated with repair (Maier et al. 2008). However, other behavioral activity patterns also occur after stroke, including compensation and learned disuse. In compensation, a subject adopts a behavioral strategy that leads to the accomplishment of a task, but is different from the strategy utilized for this task before the injury. Compensatory strategies are often inefficient, energetically expensive and may lead to long-term musculoskeletal complications (Dobkin, 2003). In learned disuse, subjects after stroke will simply not use their affected function (a limb, an aspect of language). For the reorganizing brain, this is itself a distinct activity pattern. Because axonal sprouting is a process that responds to, and is shaped by, activity (Kleim et al., 2005; Harms et al., 2009), the occurrence of both adaptive and maladaptive activity patterns will likely shape the formation of new connections after stroke. This is an interaction that will require careful study in animal models so that human clinical trials incorporate beneficial activity paradigms during the period of delivery of a putative “repair” therapy.
Future directions
Future research will be needed to address limitations in this field and problems in translation of the basic science of post-stroke axonal sprouting to the clinic. The field of neuroprotection generated consistent pre-clinical findings of mechanisms of cell death but these failed to be translated into clinical therapies. In a recent review of clinical trials in stroke, Ginsberg (2008) noted many methodological problems on both the pre-clinical and clinical sides. Many clinical trials were carried out using doses that were already known to be ineffective in preclinical trials, or time delays outside the established therapeutic window. Some trials were based on preclinical data showing relatively weak effects or that were only established in one limited model. Similar problems may occur in the field of neural repair without careful work on the key points in clinical translation (Carmichael, 2010). Within studies of post-stroke axonal sprouting, data indicate changes in some systems, but have not yet pinpointed which changes actually underlie functional recovery. Axonal sprouting occurs after stroke in peri-infarct cortex and in contralateral projections to spinal cord, brainstem and striatum. Which system, or systems, actually mediate functional recovery in their axonal sprouting response? The effective delivery of neural repair strategies is another major issue in recovery after stroke. Several growth factors and cytokines have been shown to mediate axonal sprouting or other neural repair processes such as neurogenesis (Ohab and Carmichael, 2008). However these are pleiotrophic molecules with likely multi-organ effects when delivered systemically. Selective delivery systems, or more selective small molecules, will need to be developed to minimize side-effects in a neural repair therapeutic. As shown in the work with inosine, synergistic treatments that promote axonal growth and block growth inhibitors may provide a dramatic impact on recovery after stroke. However, such combinatorial treatments require much greater work in pharmacological and toxicological testing for IND development. As also noted above, treatments that promote anatomical rewiring will need to be administered in combination with behavioral activity to help “stamp in” patterns of brain rewiring that are adaptive and to avoid the formation of maladaptive patterns of wiring. Stroke is a clinically heterogeneous disease, with infarcts commonly occurring in different tissue compartments (white matter and gray matter) and brain regions (basal ganglia, cortex, thalamus, brainstem), and occurs most often in aged individuals. Experimental stroke models usually produce infarcts in cortex or striatum in young adult rodents. A promising experimental treatment will need, at the very least, to be tested in several different rodent stroke models and aged animals. Despite these issues, it is becoming clear that the partial recovery that is commonly seen after stroke is associated with a reorganization of brain circuitry, and that methods that can safely and effectively enhance this reorganization could potentially have great clinical value.
Acknowledgments
We are grateful to the NIH (R01 NS047446 to LB; NS045729 and NS053957 to STC), the Miriam and Sheldon G. Adelson Medical Research Foundation (to LIB and STC), Alseres Pharmaceuticals (to LIB), and the Larry L Hillblom Foundation (to STC) for support of the authors' research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–70. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, et al. Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. J Biol Chem. 1998;273:29626–34. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, et al. The pattern of GAP-43 immunostaining changes in the rat hippocampal formation during reactive synaptogenesis. Brain Research Molecular Brain Research. 1990;8:17–23. doi: 10.1016/0169-328x(90)90004-w. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends in Neurosciences. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Benson MD, et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–9. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–80. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–50. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Brown CE, et al. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29:1719–34. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, et al. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–91. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, et al. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, et al. Displacement of primary sensorimotor cortex activation after subcortical stroke: a longitudinal PET study with clinical correlation. Neuroimage. 2003;19:1650–4. doi: 10.1016/s1053-8119(03)00205-2. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Translating the frontiers of brain repair to treatments: Starting not to break the rules. Neurobiol Dis. 2010 Sep 18; doi: 10.1016/j.nbd.2009.09.005. 2009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, et al. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, et al. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–22. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Chen P, et al. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–6. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, et al. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–9. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–87. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Crafton KR. Somatotopy and movement representation sites following cortical stroke. Exp Brain Res. 2006;168:25–32. doi: 10.1007/s00221-005-0082-2. [DOI] [PubMed] [Google Scholar]
- Czarnecki W, Czarnecki A. Haemodynamic effects of inosine. A new drug for failing human heart? Pharmacol Res. 1989;21:587–94. doi: 10.1016/1043-6618(89)90200-4. [DOI] [PubMed] [Google Scholar]
- Dancause N, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–79. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zoppo GJ, et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–8. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–7. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH. The Clinical Science of Neurologic Rehabilitation. Oxford University Press; New York, NY: 2003. [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian. CNS Nat Rev Neurosci. 2003;4:703–13. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fischer D, et al. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci. 2004;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani M, et al. Binding of soluble myelin-associated glycoprotein to specific gangliosides induces the association of p75NTR to lipid rafts and signal transduction. J Neurochem. 2005;94:15–21. doi: 10.1111/j.1471-4159.2005.03121.x. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, et al. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain. 2007;130:926–39. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh EL, et al. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1:10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, et al. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–4. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, et al. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–99. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–46. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grill R, et al. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–72. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, et al. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–7. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Harms KJ, et al. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J Neurosci. 2008;28:5686–5690. doi: 10.1523/JNEUROSCI.0584-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, et al. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–9. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin N, et al. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A. 2006;103:18320–5. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, et al. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–63. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, et al. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res. 1996;733:142–8. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–52. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kawamata T, et al. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A. 1997;94:8179–84. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, et al. Intracisternal antisense oligonucleotide to growth associated protein-43 blocks the recovery-promoting effects of basic fibroblast growth factor after focal stroke. Exp Neurol. 1999;158:89–96. doi: 10.1006/exnr.1999.7101. [DOI] [PubMed] [Google Scholar]
- Kleim JA, et al. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Environmental enrichment and cortical injury: behavioral and anatomical consequences of frontal cortex lesions. Cereb Cortex. 1991;1:189–98. doi: 10.1093/cercor/1.2.189. [DOI] [PubMed] [Google Scholar]
- Langley B, et al. Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke. 2009;40:2899–905. doi: 10.1161/STROKEAHA.108.540229. [DOI] [PubMed] [Google Scholar]
- Lee JK, et al. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–17. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis. 2006;23:362–73. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Liu BP, et al. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B, et al. Mst3b, an Ste20-like kinase, regulates axon regeneration in the mature. CNS and PNS Nature Neurosci. 2009;12:1403–1409. doi: 10.1038/nn.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, et al. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–9. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, et al. Postlesion axonal growth produces permanent functional connections. Science. 1973;180:1364–6. doi: 10.1126/science.180.4093.1364. [DOI] [PubMed] [Google Scholar]
- MacDonald E, et al. A novel phosphodiesterase type 4 inhibitor, HT-0712, enhances rehabilitation-dependent motor recovery and cortical reorganization after focal cortical ischemia. Neurorehabil Neural Repair. 2007;21:486–496. doi: 10.1177/1545968307305521. [DOI] [PubMed] [Google Scholar]
- Maier IC, et al. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- McGee AW, et al. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–6. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L. Ganglioside rafts as MAG receptors that mediate blockade of axon growth. Proc Natl Acad Sci U S A. 2002;99:7811–3. doi: 10.1073/pnas.132280299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–93. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, et al. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Neumann S, et al. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–93. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Ng YS, et al. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–14. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638–44. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–80. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, et al. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–41. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, et al. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex. 2006;16:529–36. doi: 10.1093/cercor/bhi132. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–52. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. A quantitative investigation of the development of collateral reinnervation after partial deafferentation of the septal nuclei. Brain Res. 1973;50:241–64. doi: 10.1016/0006-8993(73)90729-4. [DOI] [PubMed] [Google Scholar]
- Ramer MS, et al. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–6. doi: 10.1038/35002084. see comments. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, et al. Myelin-associated glycoprotein binding to gangliosides. Structural specificity and functional implications. Ann N Y Acad Sci. 1998;845:92–105. doi: 10.1111/j.1749-6632.1998.tb09664.x. [DOI] [PubMed] [Google Scholar]
- Schnell L, et al. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–3. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Scott GS, et al. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci U S A. 2005;102:3483–8. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GS, et al. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc Natl Acad Sci U S A. 2002;99:16303–8. doi: 10.1073/pnas.212645999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, et al. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–9. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Sigler A, et al. Imaging rapid redistribution of sensory-evoked depolarization through existing cortical pathways after targeted stroke in mice. Proc Natl Acad Sci U S A. 2009;106:11759–64. doi: 10.1073/pnas.0812695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–56. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Smith JM, et al. Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain. 2007;130:915–25. doi: 10.1093/brain/awl393. [DOI] [PubMed] [Google Scholar]
- Song H, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–8. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Starling RD, et al. Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med Sci Sports Exerc. 1996;28:1193–8. doi: 10.1097/00005768-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, et al. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–44. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, et al. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–93. doi: 10.1161/01.str.29.11.2381. discussion 2393-5. [DOI] [PubMed] [Google Scholar]
- Takatsuru Y, et al. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J Neurosci. 2009;29:10081–6. doi: 10.1523/JNEUROSCI.1638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrek R, et al. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–45. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–22. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. The functional anatomy of cerebral reorganisation after focal brain injury. J Physiol Paris. 2006;99:425–36. doi: 10.1016/j.jphysparis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Weidner N, et al. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–8. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J Neurosci. 2008;28:6592–606. doi: 10.1523/JNEUROSCI.0622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Yin Y, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–52. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke. 2009;40:309–12. doi: 10.1161/STROKEAHA.108.522144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai L, et al. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29:8187–97. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurn A, Do K. Purine metabolite inosine is an adrenergic neurotrophic substance for cultured chicken sympathetic neurons. Proc Natl Acad Sci USA. 1988;85:8301–8305. doi: 10.1073/pnas.85.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]


