Abstract
A retrospective case series was undertaken to evaluate nine eyes of six patients with active CMV retinitis. Patients were evaluated with a comprehensive ophthalmic examination, fundus autofluorescence imaging, and fundus photography. Oral valganciclovir, intravitreal ganciclovir, intravitreal foscarnet, or a ganciclovir implant was administered as clinically indicated.
Introduction
Cytomegalovirus retinitis is a leading cause of blindness in patients with acquired immunodeficiency syndrome (AIDS) and may also be observed in individuals with other systemic immunodeficiency syndromes, transplant recipients, and cancer patients following chemotherapy or radiation. [1–5] The diagnosis of CMV retinitis is based primarily on the appearance of characteristic hemorrhagic, full-thickness retinitis, but the presence of atypical features can sometimes make a clinical diagnosis more difficult. [6] The progression of CMV retinitis may be monitored by repeated clinical examinations or by serial fundus photographs, as used in prospective trials of oral valganciclovir and ganciclovir (GCV) implant therapy for CMV retinitis. [7, 8] Polymerase chain reaction (PCR)-based testing of aqueous humor and vitreous specimens may also be helpful in the diagnosis and monitoring of disease. [9, 10]
Clinical features of active CMV retinitis have been characterized previously using fundus photography, fluorescein angiography, optical coherence tomography (OCT), and electrophysiologic testing. [11] However, the detailed anatomical changes of the retina and retinal pigment epithelium (RPE) remain incompletely understood. Recently, fundus autofluorescence (FAF) imaging has provided an additional non-invasive in vivo method of evaluating structural changes of the RPE and outer retina. Alterations in FAF signal, arising mainly from changes in the accumulation of lipofuscin, the major fluorophore found in the retina[12], have been observed in a number of degenerative, inflammatory and infectious retinal diseases. [13–16]
In this report, we describe the clinical findings with FAF imaging in nine eyes of six patients with active CMV retinitis. In all nine eyes examined, areas of increased autofluorescence on FAF imaging (hyperautofluorescence) correlated with borders of active retinitis observed clinically. In two of six patients with only subtle clinical evidence of retinitis, FAF imaging was particularly helpful in identifying the presence and location of active disease. In another patient, widespread FAF imaging abnormalities were observed following therapy. The pathogenesis of the RPE changes following treatment is discussed here. Our findings indicate that FAF imaging may provide a useful non-invasive, adjunctive imaging technique, which may be helpful in the detection and localization of disease activity in CMV retinitis.
Methods
The medical and photographic records of six patients with active CMV retinitis from two tertiary referral centers were reviewed. All patients received a comprehensive ophthalmic examination including visual acuity, slit lamp biomicroscopy, and dilated funduscopic examination. Fundus photography with nine-field peripheral fundus photographs at the baseline evaluation, FAF imaging and OCT were also performed. For FAF imaging, the Topcon 50EX retinal camera was used with an excitation wavelength 585 nm (bandwidth 550–600) and emission wavelength 695 nm (bandwidth 660 to 800 nm). Images were obtained using OIS WinStation 5000™ software (Ophthalmic Imaging Systems, Sacramento, CA) and analyzed using Adobe Photoshop 5.5 (Adobe Systems Incorporated, San Jose, CA).
Clinical diagnoses of CMV retinitis were made based on a known history of systemic immunosuppression (i.e. HIV, immunosuppressive medications following transplantation), the presence of a hemorrhagic retinitis or retinal infiltrate, and polymerase chain reaction (PCR) testing of aqueous fluid positive for CMV DNA. Clinical improvement of disease activity was based on resolution of hemorrhagic retinitis or retinal infiltrates or the appearance of atrophic retinal pigment epithelium in areas of previously infected retina. Nine-field fundus photographs were taken in all patients who had follow-up and were able to be examined at the slit lamp. For patients who were too ill to be examined in the clinic, resolution or improvement of disease activity was based on the clinical judgment of the examining physician. Patients were treated with oral valganciclovir, intravitreal foscarnet, intravitreal ganciclovir, ganciclovir implant or a combination of the above therapies as clinically indicated. Follow-up fundus photography and FAF imaging was available in six eyes of 4 patients.
Results
Baseline patient characteristics
Nine of 12 eyes of 6 patients showed clinical evidence of active CMV retinitis. Demographic, clinical, and FAF imaging features are summarized in Table 1. The mean age of patients evaluated was 49.3 years (Range 31–73 years). The mean initial visual acuity of patients was 20/67. The mean follow-up time of 4 patients with serial FAF imaging was 4.5 months (Range 3–6 months).
Table 1.
Clinical features and FAF imaging findings
| Patient No. | Age | Gender | Eye | Medical conditions | Therapy | Initial VA | Final VA | Initial FAF features | Final FAF features | F/U (Mo.) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | OD | HIV, CNS toxoplasmosis | IVT GCV/FOS, VGCV | 20/20 | 20/20 | Hyper AF leading edge of retinitis, mixed hypo and hyper AF signal in region of prior retinitis | Few areas of decreased hyperAF at leading edge of retinitis, persistent mixed hypo and hyper AF signal in region of treated disease | 4 |
| OS | IVT GCV/FOS, VGCV | 20/25 | 20/20 | Hyper AF leading edge of retinitis, mixed hypo and hyper AF signal in region of prior retinitis | Few areas of decreased hyperAF at leading edge of retinitis, persistent mixed hypo and hyper AF signal in region of treated disease | 4 | ||||
| 2 | 35 | F | OD | HIV, CNS lymphoma | VGCV, GCV IMP | 20/50 | 20/40 | Hyper AF leading edge of retinitis, mixed hypo and hyper AF signal in region of hemorrhage and prior retinitis | NP | 2† |
| 3 | 31 | F | OD | HIV | GCV IMPL, VGCV | 20/640 | 20/200 | Hyper AF surrounding central area of hypo AF from heme and retinitis | Non-contiguous, diffuse, stippled hyper AF signal throughout posterior pole | 6 |
| 4 | 58 | F | OD | HIV | VGCV, IVT GCV/FOS, GCV IMPL | 20/200 | 20/80 | Hyper AF surrounding central area of hypo AF from heme, Stippled hyper AF peripheral to zone of active retinitis | Decreased hyper AF surrounding area of hypo AF, decreased hyper AF peripheral to zone of active retinitis | 3 |
| 5 | 73 | M | OD | LGL, MAI infection | VGCV | 20/25 | 20/25 | Hyper AF at leading edge of retinitis, mixed hypo and hyper AF anterior to border | Hyper AF at leading edge of retinitis, mixed hypo and hyper AF anterior to border | 5 |
| OS | VGCV | 20/25 | 20/25 | Hyper AF at leading edge of retinitis, mixed hypo and hyper AF signal anterior to border | Hyper AF at leading edge of retinitis, mixed hypo and hyper AF anterior to border | 5 | ||||
| 6 | 67 | F | OD | Lung TPx, SS, ILD, IMT* | VGCV, IVT FOS, GCV IMPL | 20/200 | 20/200 | Hyper AF at the leading edge of retinitis, mixed hypo and hyper AF signal anterior to border | NP | 6 |
| OS | VGCV | 20/80 | 20/100 | Hyper AF at the leading edge of retinitis, mixed hypo and hyper AF signal anterior to border | NP | 6 |
Abbreviations OD Right eye, OS Left eye, HIV Human immunodeficiency virus, CNS Central nervous system, LGL Large granular leukemia, MAI Mycobacterium avium-intracellulare TPx Transplant, SS Sjogren syndrome, ILD Interstitial lung disease, IMT*
IVT GCV/FOS Intravitreal ganciclovir/foscarnet, GCV IMPL Ganciclovir implant, VGCV Valganciclovir PO, NP Not performed, AF autofluorescent,
Patient 6 was on multiple immunomodulatory therapies including mycophenolate mofetil, prednisone, and tacrolimus
Patient died from infectious complications related to HIV/CNS lymphoma
Baseline FAF imaging features
In all 9 eyes with active CMV retinitis, FAF imaging revealed a hyperautofluorescent border that corresponded spatially to the advancing border of active retinitis as seen on fundus photography and clinical exam. In 3 eyes (patients 1 and 2) with extensive (> 10 disc diameters) peripheral involvement, mottled regions of hyper- and hypoautofluorescence were observed anterior to this hyperautofluorescent border, corresponding to regions of RPE atrophy and retinitis at various stages of healing (Figure 1, A–D). In patient 2, areas of extensive hemorrhagic retinitis also contributed to a complex pattern of peripheral autofluorescence signal, with regions of hypoautofluorescence corresponding with areas of confluent hemorrhage and active, full-thickness retinitis.
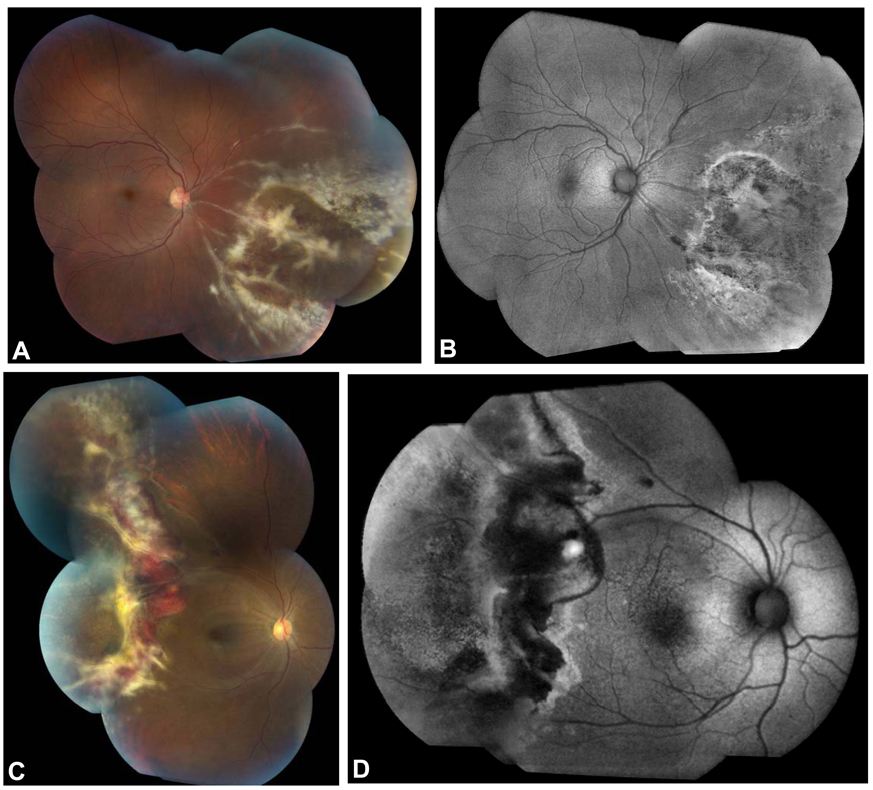
Figure 1.
Fundus photographs and corresponding FAF images of new-onset CMV retinitis in two HIV-positive patients. Fundus photograph of a 32 year-old male patient shows full-thickness retinitis in the inferonasal quadrant of the right eye (A). The FAF image demonstrated hyperautofluorescence at the posterior border of the zone of retinitis with mottled hyper- and hypoautofluorescence anterior to the advancing border in areas of clinically atrophic RPE (B). In a 35 year-old female patient with HIV and CNS lymphoma, a hemorrhagic retinitis is seen (C) and FAF imaging shows hyperautofluorescence at the posterior border of the active retinitis with a swath of hypoautofluorescence corresponding to hemorrhage, retinal edema, and full-thickness retinitis (D). Mottled hyper- and hypoautofluorescence are seen anterior to this area of active disease, corresponding to RPE atrophy seen in the fundus photograph.
In two patients (patients 3 and 4), unilateral, active CMV disease involving the macula and posterior pole was found. Active areas of CMV disease with focal hemorrhages and full-thickness retinitis corresponded to areas of hypoautofluorescence on FAF imaging (Figure 2 and Figure 3). Surrounding this zone of retinitis, a halo of hyperautofluoresence was also seen. In one patient (patient 4), there was an area of linear hyperautofluorescence, which corresponded to active CMV retinitis extension from the focal patch of retinitis (Figure 3).
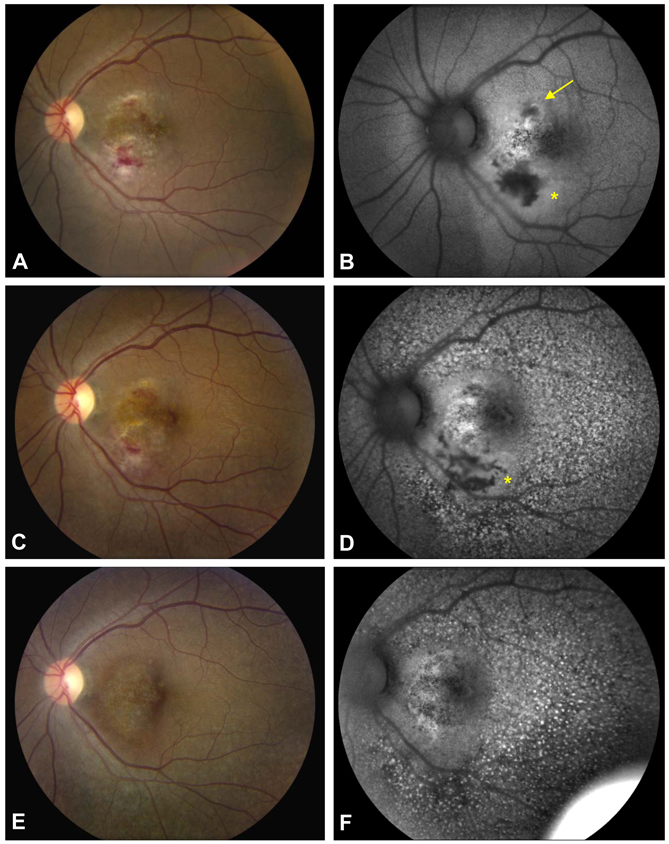
Figure 2.
Fundus photograph of the posterior pole of a HIV-positive patient with macular CMV retinitis (A) and corresponding FAF image (B) shows hypoautofluorescence with surrounding halo of hyperautofluorescence (asterisk), which corresponded to hemorrhage, retinal infiltration and retinal edema. A stippled area of hyper- and hypoautofluorescence is observed superonasal to the fovea (yellow arrow) in a smaller, non-contiguous area of active CMV retinitis. Two weeks following intravitreal foscarnet and ganciclovir, the area of active CMV retinitis has decreased (C) and the FAF image shows that the region of hypoautofluorescence has changed to an area with both hyper- and hypoautofluorescent signal (D, asterisk). Interestingly, widespread hyperautofluorescent stippling is seen on FAF imaging, which was not apparent clinically (D). At 6 months follow-up after ganciclovir implantation, the CMV retinitis has resolved (E), however, diffuse and persistent FAF abnormalities are seen, possible related to medication toxicity (F).
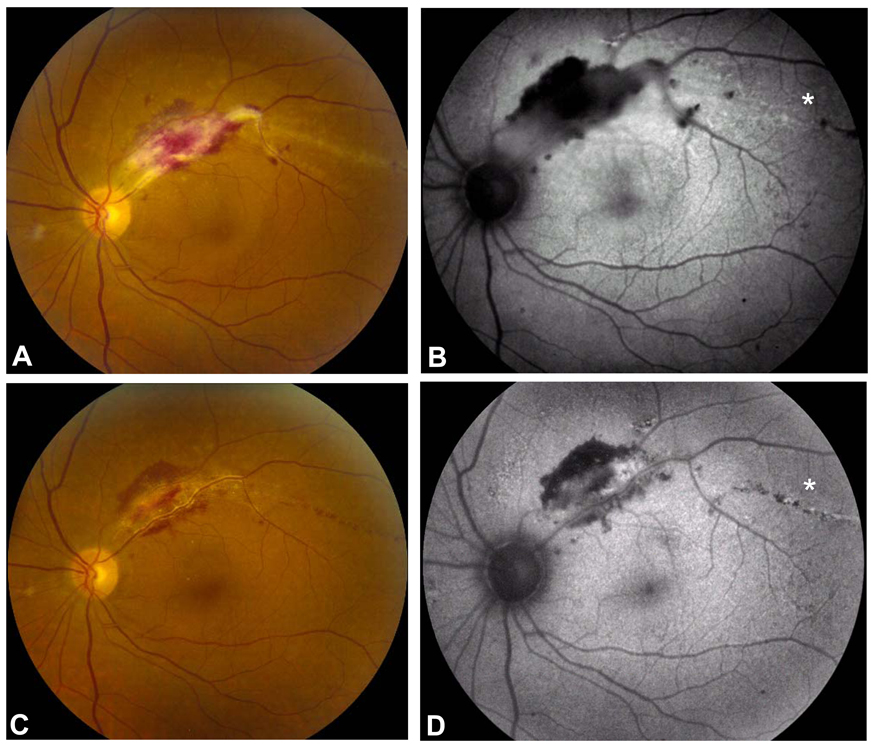
Figure 3.
Fundus photograph OS of a HIV-positive patient with CMV retinitis involving superotemporal macula with a serous retinal detachment (A) and corresponding FAF image showing hypoautofluorescence with a faint halo of hyperautofluorescence in the zone of hemorrhagic retinitis and retinal edema (B). Hyperautofluorescent stippling is also seen temporally (asterisk), and corresponds to a linear streak of advancing infection. Following intravitreal ganciclovir and foscarnet injections, the CMV retinitis began to resolve (C) with a corresponding decrease in the density of the hypoautofluorescence at the superotemporal arcade (D). The prior area of linear hyperautofluorescence now shows linear hyper- and hypoautofluorescence (asterisk). Clinically, this area of CMV retinitis is also resolving and has been replaced by punctate hemorrhages suggestive of small-vessel injury during the resolution phase of the disease.
Patients 5 and 6 who had both been on chronic, suppressive doses of oral valganciclovir therapy (450 mg bid) for recurrent CMV disease presented with subtle, bilateral retinitis and edema at the posterior border of an area of prior CMV retinitis. In both patients, FAF imaging revealed hyperautofluorescence at the border of active retinitis, which was much more prominent on FAF imaging than that appreciated clinically, and assisted in the diagnosis of a CMV retinitis recurrence (Figure 4). PCR evaluation of aqueous humor specimens from both of these patients confirmed high copy numbers of CMV DNA, supporting the diagnosis of active CMV retinitis. Following the identification of active retinitis, valganciclovir was increased to the treatment dosage (i.e. 900 mg bid) in both of these cases. The medical history for both of these patients was notable in that both did not have HIV-related immunosuppressive disease. Patient 5 had a history of possible large granular leukemia and patient 6 was taking mycophenolate mofetil, tacrolimus, and prednisone following lung transplantation for interstitial lung disease. Both of these patients also demonstrated stippled areas of hyper- and hypoautofluorescence anterior to the border of advancing retinitis, which were correlated spatially with areas of RPE atrophy from prior CMV retinitis.
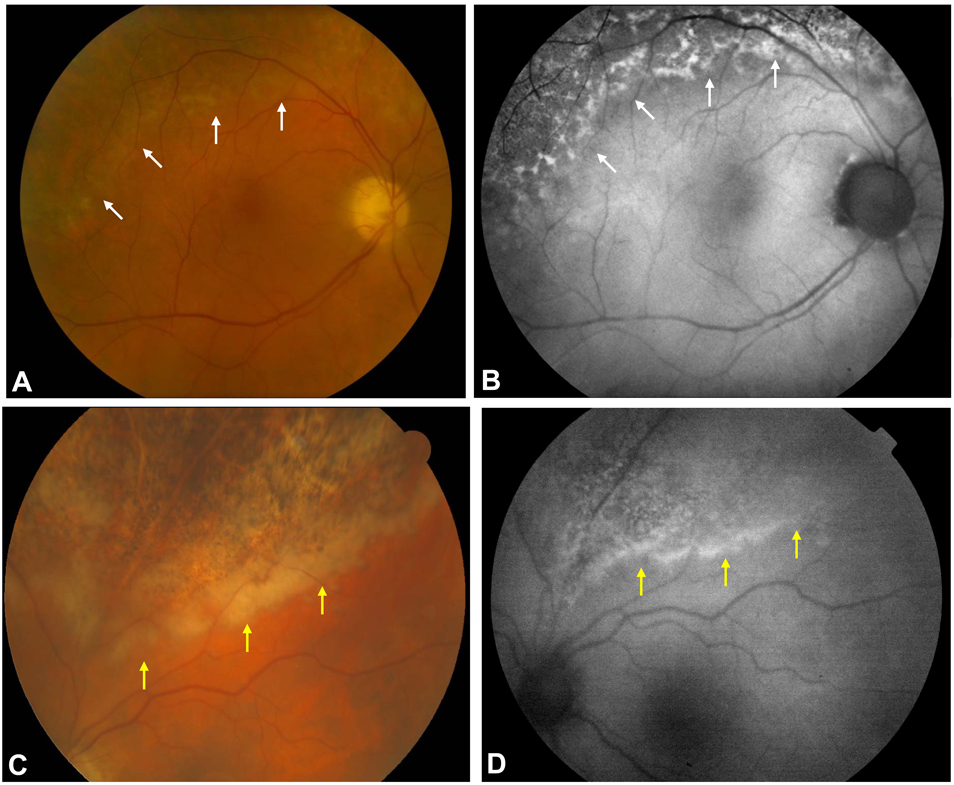
Figure 4.
Fundus photograph of 73 year-old patient with large granular leukemia and a history of prophylactic valganciclovir shows subtle retinal whitening at he border of prior CMV retinitis and RPE atrophy (A, white arrows). FAF imaging reveals hyperautofluorescence in the area of the CMV retinitis recurrence (B, white arrows), which was confirmed by PCR testing. A 67-year-old patient who was status post lung transplantation and on multiple immunosuppressive medications and prophylactic valganciclovir complained of new floaters in both eyes. Fundus photography showed retinal whitening at the posterior border in the area of prior CMV retinitis (C, yellow arrows). FAF imaging highlighted this border of active CMV retinitis with hyperautofluorescence corresponding to the area of CMV retinitis recurrence (yellow arrows, D). PCR testing confirmed the presence of CMV DNA and led to the institution of valganciclovir at higher treatment doses.
FAF imaging after therapy
Of the patients with CMV retinitis evaluated with FAF imaging, we were able to obtain serial FAF imaging in 6 eyes of 4 patients to evaluate for changes in FAF patterns during the evolution of resolving disease activity. Four eyes of three patients demonstrated fading of hyperautofluorescence at the border of active CMV retinitis (patients 1, 3, and 4). Both eyes of patient 5 demonstrated persistence of abnormal hyperautofluorescence. Fundus autofluorescence could not be performed on patients 2 and 6 due to systemic illness requiring hospitalization. All patients improved clinically while on anti-viral therapy. Thus, it is not clear whether a change in autofluorescent signal was a reliable indicator of disease regression from this small series of patients evaluated.
In the two patients with unilateral macular CMV retinitis (patients 3 and 4), central zonal hypoautofluorescence surrounded by hyperautofluorescence was seen in the area of active retinitis on initial exam. Following therapy, this area of hypoautofluorescence became more heterogeneous and the rim of hyperautofluorescent signal appeared to dissipate in both patients (Figure 2 and Figure 3). Interestingly, in one patient (patient 3), diffuse, stippled hyperautofluorescence was observed following intravitreal ganciclovir and foscarnet injections, likely representing lipofuscin accumulation within RPE cells or RPE injury (Figure 2). This diffuse change in the FAF appearance involving both the posterior pole and mid-peripheral retina persisted at 6 month follow-up. The mechanism for this finding was unclear; however, given its temporal relationship to the intravitreal anti-viral injections, it is possible that medication toxicity played a role.
Discussion
The characteristic clinical appearance of CMV retinitis in the setting of HIV/AIDS or other immunosuppressed conditions makes the diagnosis straightforward in the majority of cases. However, in subtle or atypical cases, diagnosis and disease monitoring can prove to be challenging. In current practice, follow-up examinations to monitor for CMV progression consist of clinical evaluation and fundus photography, although PCR-based techniques have also been used. While systemic antivirals and the local delivery of antiviral medications have demonstrated efficacy in the treatment of CMV retinitis, the progressive tissue destruction and atrophy following an episode of CMV retinitis may culminate in retinal detachment and high visual morbidity. In this context, non-invasive testing techniques such as FAF imaging may help to detect subtle RPE dysfunction that may herald disease recurrence and aid in the understanding of structural changes occurring in affected patients.
The patterns of autofluorescence observed in this series of patients are suggestive of disruption of the structural integrity of the RPE at the border of active retinitis. In all 9 eyes of 6 patients with active CMV retinitis, hyperautofluorescence was observed at the advancing border of active CMV retinitis. Follow-up FAF imaging differed between patients who demonstrated clinical improvement. Specifically, four eyes of three patients showed fading of hyperautofluorescence on follow-up FAF imaging while both eyes of one patient demonstrated ther persistence of hyperautofluorescence signal despite apparent clinical improvement. Thus, the utility of FAF imaging is valuable in the acute monitoring of CMV disease activity while patients are undergoing anti-viral therapy will require further study.
In two patients with predominantly posterior pole involvement, central hypoautofluorescence with a surrounding halo of hyperautofluorescence was correlated with areas of active, hemorrhagic retinitis surrounded by a zone of retinal edema. The central hypoautofluorescent signal may be due to RPE death or possibly blockage of intrinsic fundus autofluorescence from hemorrhage and overlying edema. It is interesting to note that this hypoautofluorescent signal became more heterogeneous over time with patchy areas of both hyper- and hypoautofluorescence. It is possible that as the edema, hemorrhage and active infectious processes are eradicated with anti-viral therapy, underlying RPE injury and engulfed photoreceptor degradative byproducts are better visualized with FAF imaging.
The FAF findings of patient 3 was notable in that the diffuse, punctate areas of hyperautofluorescence were visualized more readily on FAF imaging than on clinical exam, and these areas appeared to be independent of the active retinitis. The disparate locations of the area of resolving retinitis and widespread RPE and FAF abnormalities make it unlikely that this process was infectious in origin. Because of the temporal relationship of the change in abnormal FAF signal with the intravitreal foscarnet injection, direct RPE toxicity or a local retinotoxic effect with secondary RPE disruption may have been occurred. This process has not been observed in other patients who have received intravitreal foscarnet or ganciclovir injections, but further studies to evaluate this unusual finding are needed.
In two patients (patients 5 and 6), the FAF imaging features were more prominent than the changes on observed clinical exam. Both patients were notably on long-term prophylactic doses (> 6 months) of valganciclovir with multiple bouts of CMV retinitis. Their presentation differed from the HIV-positive patients in that they had both previously received anti-viral therapies for CMV retinitis. Their clinical presentation likely represented either partially treated disease or early CMV reactivation, and PCR studies on aqueous fluid confirmed the presence of CMV DNA in high copy numbers. In both patients, FAF imaging was helpful in identifying and localizing the area of active disease.
Pathologic studies of CMV retinitis have described a necrotizing retinitis with discrete advancing edges and involvement of both the sensory retina and retinal pigment epithelium.[17] In one prior study of CMV retinitis treated with sustained-release intravitreal ganciclovir device, electron microscopy showed the presence of virus particles at the junction between uninvolved and healed retinitis. [18] In immunohistochemical studies evaluating regions of full-thickness necrotizing retinitis, CMV antigens have been identified mostly within Muller cells and perivascular glial cells with some focal staining of RPE cells and neuronal cells. [19] Interestingly, in areas adjacent to the regions of full-thickness necrosis, CMV antigen was identified within vascular endothelial cells. It has been hypothesized that CMV infection of viral endothelial cells may lead to secondary involvement of neuronal and glial cells and subsequent disruption of RPE integrity. [19] Besides the potential disruption of RPE integrity from surrounding CMV infection and inflammation, CMV may also primarily infect RPE cells via endocytosis and has been found to replicate within RPE cells.[20–22]
Inflammatory processes following CMV infection of vascular endothelial cells, perivascular glial cells and Muller cells may contribute to the hyperautofluorescent signal via engulfment of cellular degradation products by the RPE. Moreover, microglia may also accumulate photoreceptor outer segment debris in conditions of inflammation, leading to additional autofluorescent signal.[23] In age-related macular degeneration, A2E (N-retinylidene-N-retinylethanol-amine) is thought to be the dominant fluorophore found in lipofuscin. Other products thought to contribute to fundus autofluorescence include precursors to A2E, compounds comprised of molecules derived from retinoids and other constituents besides ethanolamine, and other peroxidation products derived from lipids and proteins. [20] In infectious processes such as CMV retinitis, the major fluorophores from which autofluorescence signals are derived are unknown, but likely differ from those found in age-related macular degeneration.
Potential limitations of this retrospective, observational case series include the small number of patients evaluated, limited follow-up, and the unmasked nature of the evaluation of autofluorescence imaging and clinical fundus photographs. A potential future study would involve a masked review of patients with active and inactive CMV retinitis with clear definitions for clinical disease activity, disease progression, or disease resolution. Longer follow-up would also be helpful to determine the course of hyperautofluorescent signal, which was persistent in one patient following treatment of active CMV retinitis. A control group with healed CMV retinitis lesions for comparison would also be helpful in determining the long-term autofluorescent changes that are observed in CMV retinitis. The unmasked nature of the comparison of clinical fundus photographs and FAF imaging potentially could introduce bias on the part of the investigators; however, the appearance of hyperautofluorescent borders in active CMV retinitis was consistently identified in several patients, and this imaging modality in the evaluation of CMV retinitis warrants further investigation.
The FAF imaging features described in this series of patients with active CMV retinitis suggest that additional pathogenic processes occur at the level of RPE, changes which are likely related to infectious and inflammatory processes involving neural retina, vascular endothelial cells, and glial cells. We hypothesize that phagocytosis of photoreceptor cellular debris by RPE cells or microglia may lead to the abnormal FAF signals seen in this series. FAF imaging appears to provide useful information in identifying patients with active CMV retinitis and potentially in monitoring patients for disease recurrences. Whether the FAF imaging features of patient 3 represent RPE toxicity remains to be elucidated. Further studies are needed to determine if FAF imaging may be utilized as an adjunct to clinical examination, fundus photography, and PCR-based testing for the evaluation of CMV retinitis or other herpetic viral retinitides.
Acknowledgements
This research is supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health. Dr. Steven Yeh has received support from the Heed Ophthalmic Foundation.
Footnotes
This research was presented in part, as a paper presentation at the American Society of Retinal Specialists Annual Meeting in Maui, HI in October 2008.
References
- 1.Nussenblatt R. Ocular complications of the acquired immunodeficiency syndrome. Nat Immun Cell Growth Regul. 1988;7(3):131–134. [PubMed] [Google Scholar]
- 2.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 3.Church J, et al. Cytomegalovirus retinitis in chronic lymphocytic leukaemia. Eye. 2007;21(9):1230–1233. doi: 10.1038/sj.eye.6702550. [DOI] [PubMed] [Google Scholar]
- 4.Long HM, Dick A. Presumed CMV associated necrotizing retinopathy in a non- HIV immunocompromised host. Clin Experiment Ophthalmol. 2005;33(3):330–332. doi: 10.1111/j.1442-9071.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- 5.Perren BA, et al. Cytomegalovirus retinitis and optic neuritis in a child with severe combined immunodeficiency syndrome. Retina. 1996;16(2):117–121. doi: 10.1097/00006982-199616020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Davis JL. Differential diagnosis of CMV retinitis. Ocul Immunol Inflamm. 1999;7(3–4):159–166. doi: 10.1076/ocii.7.3.159.4006. [DOI] [PubMed] [Google Scholar]
- 7.Martin DF, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch Ophthalmol. 1994;112(12):1531–1539. doi: 10.1001/archopht.1994.01090240037023. [DOI] [PubMed] [Google Scholar]
- 8.Martin DF, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 9.Rothova A, et al. Usefulness of Aqueous Humor Analysis for the Diagnosis of Posterior Uveitis. Ophthalmology. 2007 doi: 10.1016/j.ophtha.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Matos K, et al. Correlation between clinical diagnosis and PCR analysis of serum, aqueous, and vitreous samples in patients with inflammatory eye disease. Arq Bras Oftalmol. 2007;70(1):109–114. doi: 10.1590/s0004-27492007000100020. [DOI] [PubMed] [Google Scholar]
- 11.Harrison JM, van Heuven WA. Retinal pigment epithelial dysfunction in human immunodeficiency virus-infected patients with cytomegalovirus retinitis. Ophthalmology. 1999;106(4):790–797. doi: 10.1016/S0161-6420(99)90168-9. [DOI] [PubMed] [Google Scholar]
- 12.Delori FC, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36(3):718–729. [PubMed] [Google Scholar]
- 13.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42(8):1855–1866. [PubMed] [Google Scholar]
- 14.Yannuzzi LA, et al. Ophthalmic fundus imaging: today and beyond. Am J Ophthalmol. 2004;137(3):511–524. doi: 10.1016/j.ajo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Holz FG. [Autofluorescence imaging of the macula] Ophthalmologe. 2001;98(1):10–18. doi: 10.1007/s003470170194. [DOI] [PubMed] [Google Scholar]
- 16.Framme C, et al. Fundus autofluorescence in APMPPE in association with lyme disease. Retina. 2002;22(5):653–657. doi: 10.1097/00006982-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Chumbley LC, et al. Adult cytomegalovirus inclusion retino-uveitis. Am J Ophthalmol. 1975;80(5):807–816. doi: 10.1016/0002-9394(75)90276-7. [DOI] [PubMed] [Google Scholar]
- 18.Anand R, et al. Pathology of cytomegalovirus retinitis treated with sustained release intravitreal ganciclovir. Ophthalmology. 1993;100(7):1032–1039. doi: 10.1016/s0161-6420(13)31524-3. [DOI] [PubMed] [Google Scholar]
- 19.Rao NA, Zhang J, Ishimoto S. Role of retinal vascular endothelial cells in development of CMV retinitis. Trans Am Ophthalmol Soc. 1998;96:111–123. discussion 124-6. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, et al. Human cytomegalovirus uses two distinct pathways to enter retinal pigmented epithelial cells. Proc Natl Acad Sci U S A. 2007;104(50):20037–20042. doi: 10.1073/pnas.0709704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodaghi B, et al. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Invest Ophthalmol Vis Sci. 1999;40(11):2598–2607. [PubMed] [Google Scholar]
- 22.Detrick B, et al. Cytomegalovirus replication in human retinal pigment epithelial cells. Altered expression of viral early proteins. Invest Ophthalmol Vis Sci. 1996;37(5):814–825. [PubMed] [Google Scholar]
- 23.Xu H, et al. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7(1):58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]