Abstract
The recent identification of tumor-initiating colorectal cancer (CRC) stem cells in the pathogenesis of CRC has provided a potential target for novel therapeutics. Many details about CRC stem cells, however, remain poorly understood. Several potential markers of CRC stem cells have been proposed, including CD133, CD44, and, recently, Lgr5. Attention also has been drawn to control of stem cell self-renewal, proliferation, and differentiation by the Wnt and transforming growth factor (TGF)-β pathways. Disruption of Wnt signaling, via loss of APC (adenomatous polyposis coli), is among the earliest events in the multistage progression of CRC and likely occurs in basal crypt stem cells, generating a neoplastic cell population that then expands upward to occupy the rest of the crypt. TGF-β signaling is a key tumor suppressor pathway, and mutations in the type II receptor and Smad4 are observed in CRC specimens and are associated with more aggressive disease in tumors with disrupted Wnt signaling. Loss of the TGF-β adaptor protein β2-spectrin is associated with loss of colonic cell polarity and architecture, and its expression parallels that of Smad4. This review suggests rational approaches to target CRC stem cells as a novel and effective way to treat advanced and difficult-to-treat CRC.
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and women and the second leading cause of cancer-related death in the United States, with an estimated 108,070 newly diagnosed cases and 49,960 deaths in 2008 [1]. The lifetime risk of developing the disease is as high as 6% in the American population [2]. Colorectal tumors arise through a multistep progression involving the mutational activation of oncogenes coupled with the mutational inactivation of tumor suppressor genes, leading to development of an adenoma and progressing to a malignant carcinoma [3]. Mutations in at least four or five genes are required for formation of a malignant tumor, and although genetic alterations often occur in a preferred sequence, the total accumulation of changes determines the final tumor phenotype [4].
Much of our understanding of the molecular pathogenesis of CRC has come from study of heritable forms of colon cancer. Although colon cancer is primarily sporadic, heritable forms of colon cancer constitute 5% to 10% of all colon cancer cases. Familial adenomatous polyposis (FAP) is an autosomal dominant CRC syndrome caused by a mutation in the tumor suppressor gene APC (adenomatous polyposis coli). APC encodes a protein that is part of a complex that binds β-catenin, targeting it for degradation. In the absence of binding and degradation of β-catenin by this complex, β-catenin translocates to the nucleus and activates multiple transcription factors, including cyclin D1 and c-myc, responsible for proliferation, differentiation, migration, and apoptosis of cells [5••]. Additional mutations, including mutation of KRAS and TP53, and deletion on chromosome 18q are required for subsequent tumor progression [6]. Hereditary nonpolyposis colon cancer or Lynch syndrome, meanwhile, involves a germline mutation in one of the DNA mismatch repair (MMR) genes, leading to defective DNA repair.
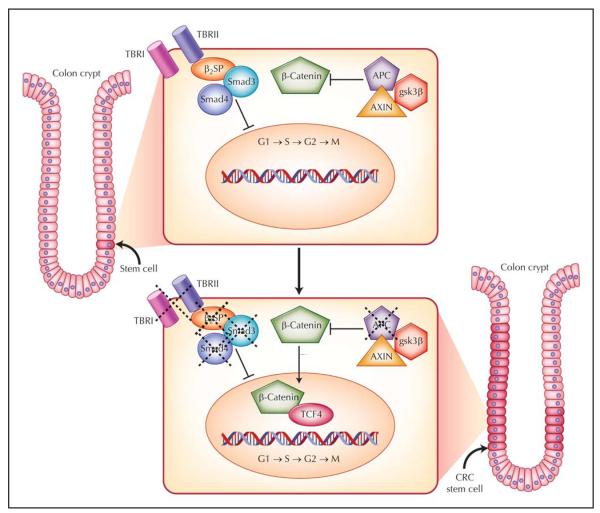
The scope of CRC and the resistance of some tumors to standard chemotherapeutic agents and radiation have brought increased focus on the hypothesis that CRC is driven by transformed cancer stem cells (Fig. 1) [5••]. Recently, much evidence points to the existence of CRC stem cells in the pathogenesis of CRC. First, stem cells are the only cells that persist long enough to undergo the requisite number of changes described in the multistage progression of CRC. Second, cancers originate in a select cell population from normal tissue stem/progenitor cells with disrupted self-renewal. Self-renewal, a defining characteristic of stem cells, is a cell division in which one or both daughter cells remain undifferentiated, thereby retaining the ability to give rise to another stem cell with the same capacity to proliferate. Stem cells also have the capacity to differentiate, thereby generating organ-specific cells. Cancer stem cells have the ability to self-renew, a characteristic that drives tumorigenesis, and to aberrantly differentiate, a property that generates the bulk of cells within a tumor [5••]. In CRC, disrupted stem cell self-renewal and differentiation have been linked to several key signaling pathways, including Wnt and transforming growth factor (TGF)-β.
Figure 1.
The colorectal cancer (CRC) stem cell model. In the normal state, stem cells are located near the base of the crypt. Inactive Wnt signaling and activated transforming growth factor (TGF)-β signaling regulate cell growth and mitigate overactivation of cell cycle–related target genes. In hereditary colon cancer syndromes such as familial adenomatous polyposis, the Wnt signaling pathway is activated secondary to a functional abnormality in the adenomatous polyposis coli (APC) protein. The defect in this protein permits β-catenin to translocate to the nucleus to activate downstream transcription factors and growth-related targets. This likely occurs in CRC stem cells, resulting in the generation of many “preneoplastic” colon polyps. Disruption of TGF-β signaling via loss of β2-spectrin (β2SP), Smad4, or the type II receptor (TBRII) synergistically promotes CRC tumorigenesis, likely in stem cells. gsk3β—glycogen synthase kinase 3β; TBRI—type I receptor.
The identification of CRC stem cells also has focused research on developing novel therapies specifically targeting these cells. Recent advances in translational research have brought significant progress in the diagnosis, prevention, and treatment of CRC. Oncologists now possess a variety of systemic drugs capable of inducing tumor responses in patients with CRC. Advanced colorectal tumors, however, often are resistant to these cytotoxic systemic therapies. In this review, we highlight the location and identification of CRC stem cells using unique biomarkers, the unique microenvironment or niche within which they reside, and several key pathways disrupted in the malignant transformation of colorectal stem cells. These may serve as potential targets in developing novel therapeutics specifically targeting CRC stem cells and hold promise for truly curative approaches to patients with advanced CRC.
Colonic Stem Cell Niche
The epithelial layer of the human colon is a dynamic and rapidly proliferating system replaced every 5 days. It consists of a single sheet of columnar epithelial cells, which form finger-like invaginations into the underlying connective tissue of the lamina propria, called crypts of Lieberkühn, each of which contains 2000 to 3000 cells [6]. Colonic stem cells are believed to be located toward the base of the crypt and are surrounded by mesenchymal cells to form a stem cell niche. The mesenchymal cells are of the myofibroblast lineage and are closely associated with the basal lamina surrounding the crypt. Pericryptal myofibroblasts produce several factors, including Wnt signaling ligands and the bone morphogenetic protein antagonists gremlin 1 (GREM1) and GREM2, that along with the Notch signaling pathway and the effects of Wnt signaling on ephrin B1 and its receptors EphB2 and EphB3, regulate maintenance of the undifferentiated stem cell and affect cell migration and differentiation [7-9].
Via asymmetric cell division, colonic stem cells undergo self-renewal and generate a population of transit cells that then migrate up the crypt, proliferating and differentiating to form the four major terminally differentiated epithelial cell types of the colon: colono-cytes, goblet cells, endocrine cells, and Paneth cells. This “unitarian theory” that all the cell types originate from a single multipotential stem cell through several committed progenitors was first formulated in 1974 and has since been verified experimentally [10,11]. The self-renewing stem cell population is responsible for colonic epithelial cell renewal and is long-lived, continuing to perform this task over the lifetime of an individual [12].
Colonic Stem Cell Markers
In the search for the location of intestinal stem cells, several molecules have been proposed as potential markers, including musahi-1 (Msi-1), DCAMLK1 (also known as DCLK1), β1 integrin subunit, EphB receptors, and most recently, Lgr5 [12-16,17•]. The evidence supporting many of these markers, however, is minimal and based primarily on the finding of a small number of positively labeled cells in the putative stem cell position toward the bottom of the crypt. Specifically, Msi-1 is an RNA-binding protein thought to be involved in asymmetric cell division during Drosophila melanogaster neural development. Immunohistochemical analysis of normal human colonic crypts demonstrated positive labeling for Msi-1 in 19.0 − 7.53 cells located in the lower region of the crypt [13]. Similarly, fluorescence analysis of frozen sections of colonic mucosa demonstrated that β1 integrin subunits localize to the lower portion of the crypt and EphB receptors are expressed in a gradient manner, with highest expression at the bottom of the crypt and lowest expression at the crypt–villus junction [9,18]. Expression of both EphB2 and EphB3 receptor has been reported in the lower crypts of mouse colon, and inhibition of EphB2/EphB3 signaling demonstrated a reduction in the number of crypt cells expressing markers of proliferation. Moreover, constitutive overactivation of EphB2 receptor signaling correlated with an increase in the number of proliferating crypt cells when compared with wild-type intestine. It subsequently was shown that these receptors promote cell cycle reentry, thereby regulating cell proliferation [16]. However, the use of EphB receptor expression as an independent marker of colonic stem cells is still inconclusive and requires further analysis.
Recently, experimental approaches have used the ability of stem cells, once clonally marked, to repopulate the crypt with clonal descendents in all cell lineages to demonstrate multipotentiality. Lgr5 (also known as Gpr49), an intestinal Wnt target gene that encodes a G protein–coupled receptor characterized by a large leucine-rich extracellular domain and seven transmembrane domains, was identified recently as a potentially specific intestinal stem cell marker. Using in situ hybridization, it was shown to be exclusively expressed in cycling columnar cells at the very base of the crypt. These results were confirmed with the fate mapping of Lgr5-positive cells using an R26R-lacZ/Cre reporter strain, demonstrating that Lgr5-positive crypt base columnar cells generated all epithelial lineages over a 60-day period [17•,19]. Additionally, Lgr5-GFP mice were then intercrossed with Apcmin mice to examine expression of Lgr5 in premalignant adenomas, which spontaneously arise in the intestines of these transgenic mice as a result of chronic activation of the Wnt signaling pathway. Lgr5-GFP expression was restricted to a few cells within large adenomas, suggesting that Lgr5 may mark not only normal intestinal stem cells, but also a limited population of cancer stem cells [19].
Several other molecules also have been proposed as markers of CRC stem cells, including CD133 [20,21], CD44 [21-23], CD34 [24], CD24 [22,23], and epithelial-specific antigen (ESA) [22,23,25•]. Studies have demonstrated that injecting cancer stem cell–enriched populations into immunocompromised mice at low concentrations results in the formation of tumors with histology and phenotypic heterogeneity equivalent to those of the original neoplasm, whereas injecting noncancer stem cells, even at high concentrations, results in the growth of few or no tumors. In particular, three recent studies evaluating the functionality of specific biomarkers using a combination of flow cytometric analysis to identify a stem cell population and xenograft modeling to determine the tumor initiation potential of the cellular fraction have provided compelling evidence for the existence of CRC stem cells. In the first study, CD133+ cells were isolated from seven primary colon cancers and 10 extracolonic (metastatic) sites. The percentage of CD133+ cells in the tumorigenic population ranged from 3.2% to 24.5%, compared with matching normal tissues, in which the percentage of CD133+ cells ranged from 0.4% to 2.1%. CD133+ cells were then transplanted into the renal capsule of NOD/SCID mice and readily developed tumors displaying morphologic features equivalent to those of the parental tumor. In comparison, CD133− cells and nonsegregated populations did not induce tumor formation. Tumor phenotype was further maintained upon serial transplantation [26•]. Similarly, in the second study CD133+ cells were isolated from colon cancer specimens and perpetuated in vitro as floating colonies or “tumor spheres.” These tumor spheres were enriched in a tumorigenic population and maintained in serial passages [27•]. Meanwhile, in the third study, CD44 and ESA were used as stem cell–specific markers, with further enrichment by CD166. Isolated cells were then injected into NOD/SCID mice, and in each case, as few as 200 to 500 cells were needed to reconstitute a tumor phenotypically similar to the original colon cancer. In contrast, as many as 10,000 of the ESAlow/CD44− cells could not form a tumor [25•].
Significant controversy exists, however, over the functional significance of these markers of CRC stem cells. An examination of 12 colon cancer cell lines demonstrated that injections of up to 10,000 CD133+ or CD133− cells into nude mice gave rise to tumors that could be serially transplanted. CD133+ cells, however, did lead to tumors with decreased latency [28]. Further studies using a transgenic mouse model in which the CD133 promoter drove lacZ reporter expression demonstrated that CD133 was expressed by both mature and undifferentiated colonic epithelial cells, suggesting that CD133 is not a specific stem cell marker. Subsequent studies using the interleukin (IL)-10 null mouse, which is characterized by chronic intestinal inflammation correlating with neoplastic growth, demonstrated CD133 reporter construct expression throughout the entire epithelium. Furthermore, in primary human colon cancer specimens, CD133 was expressed in most of the tumor cell population and sorting of CD133+ cells from metastatic colon cancer to liver demonstrated that CD133 high- and low-expressing cells could generate tumors in NOD/SCID mice. In fact, compared with CD133+ cells, the CD133− cells gave rise to tumors with decreased latency [29•]. Therefore, further evaluation of biomarkers of CRC stem cells is needed and must be tied to functional studies demonstrating specificity and reproducible tumorigenesis on serial transplantation.
Regulation of Colonic Stem Cell Function by the Wnt Signaling Pathway
The isolation of potential CRC stem cells also has led to a significant focus on signaling pathways disrupted in stem cell self-renewal, proliferation, and differentiation. One of the most important pathways for the control of intestinal epithelial stem cell function and carcinogenesis is the Wnt/β-catenin signaling pathway, as evidenced by a germline mutation in the APC gene in FAP. APC mutations are found even in 75% to 80% of sporadic CRCs. Wnt genes encode a large family of secreted proteins with important roles in cell fate and proliferation. In humans, there are at least 19 members of the Wnt family and 10 members of its receptor family Frizzled (Fz) [30]. The Wnt receptor complex is composed of Fz plus LRP5 and LRP6 (low-density protein receptor–related proteins). Binding of Wnt to Fz activates one of two pathways: the canonical pathway, which involves β-catenin and controls cell proliferation, or the planar pathway, which involves Ca2+ and is important in cellular movement and polarity [31]. In the canonical pathway, in the absence of Wnt, cytosolic β-catenin, which is normally bound to membranous E-cadherin, interacts with a complex consisting of the tumor suppressor protein APC, glycogen synthase kinase 3β (gsk3β), and axin. β-Catenin consequently is serine phosphorylated, recognized by an E3 ubiquitin ligase, and then degraded [30]. In the presence of Wnt binding to Fz, however, the kinase activity of the APC/gsk3β/axin complex is blocked and β-catenin remains unphosphorylated and accumulates in the nucleus. β-Catenin then binds to the transcription factor TCF4 and can activate downstream target genes such as cyclin D1 and c-myc, proto-oncogenes that promote entry of the cell into the S phase of the cell cycle [32].
β-Catenin also has an important role in cell movement, polarity, and adhesion. It joins with α-catenin to anchor E-cadherin’s short cytoplasmic domain to actin filaments so that the extracellular cadherin domain can form a tight Ca2+-dependent linkage (adherens junction) to neighboring colon cells [33]. Any unattached β-catenin is subsequently degraded by the APC/gsk3β/axin complex.
Calcium serves as an important signal for transit-amplifying cells of the colon to stop proliferating and to differentiate. This signal is mediated by Ca2+-sensing receptors (CaSRs), leading to production of Ca2+-calmodulin and activation of Ca2+/calmodulin-dependent kinase II (CaMKII) and CaMKIV, which further stimulate increased CaSR expression. The CaSR triggers a shift from colon cell proliferation to terminal differentiation by inducing expression of E-cadherin and increasing expression of APC, thereby terminating β-catenin–mediated cell proliferation. The surge in APC also suppresses antiapoptosis-surviving expression, leading to a loss of resistance to apoptosis in colon cells migrating up the crypt [34].
Recent evidence demonstrates that the β-catenin/TCF4 pathway also plays a key role in maintaining intestinal progenitor cells in adult mammalian crypts. Using human colon adenocarcinoma cell lines with an inducible dominant-negative TCF4 mutation, studies have demonstrated that inhibition of β-catenin/TCF4 complex formation leads to halted growth in G1 phase of the cell cycle and induction of an intestinal differentiation program. Therefore, the β-catenin/TCF4 complex may act as a master switch that controls the balance between proliferation and differentiation in healthy and malignant intestinal epithelial cells (Fig. 1) [18,30]. Similarly, studies using transgenic expression of the secreted Wnt inhibitor Dickkopf-related protein 1 (Dkk-1) in mouse intestine demonstrated a phenotype similar to the TCF4-knockout mouse, with a reduction in the number of proliferating cells within the crypts of neonates and the complete loss of crypts in adult animals [35].
Moreover, Wnt pathway members are differentially expressed along the crypt axis and in the pericryptal mesenchymal cells forming the colonic stem cell niche. Specifically, mRNA for secreted Fz-related protein (sFRP)-5, Wnt-3, Wnt-6, Wnt-9b, and Fz-5 proteins is detected within epithelial cells near the bottom of the crypt and decreases in concentration toward the surface and more differentiated cells. Pericryptal mesenchymal cells also have been found to express sFRP-1, whereas epithelial cells located toward the crypt surface have been found to express the Wnt inhibitor Dkk-3 [36].
The links among colonic stem cells, Wnt signaling, and CRC tumorigenesis are most evident with mutations in the APC gene. Loss of APC is among the earliest events in the multistage progression of CRC. The earliest identifiable colonic lesions, aberrant crypt foci, already bear APC mutations. APC loss is also the primary mutation in FAP, which is characterized by the development of hundreds to thousands of adenomatous polyps. Recent studies suggest that mutation of the APC gene occurs in the basal crypt colonic stem cells and the resulting neoplastic population then expands upward from this position to occupy the rest of the crypt in a “bottom-up” fashion (Fig. 1). An examination of human small colorectal adenomas and tumors from patients with FAP demonstrated nuclear β-catenin accumulation in dysplastic cells emanating from the base of the crypt. Toward the surface of the crypts, there was a demarcation between dysplastic cells with nuclear β-catenin and phenotypically normal surface epithelial cells near the lumen with membrane-bound β-catenin. Using serial sections, investigators demonstrated this phenomenon within a single crypt, thereby suggesting that dysplastic cells were not spilling out over the top of one crypt into another, but developed from aberrant stem cells at the crypt base [37]. Moreover, tumor specimens from FAP patients also have shown an increased labeling index at the bottom of the crypt and a marked increase in the crypt fission rate compared with control mucosa, suggesting increased numbers or activity of colonic stem cells [38].
The Wnt signaling pathway clearly is a key player in colonic stem cell proliferation, differentiation, and transformation to CRC stem cells, making it an attractive target for novel therapeutics.
Regulation of Colonic Stem Cell Function by the TGF-β Signaling Pathway
Another key pathway in intestinal stem cell function and carcinogenesis is the TGF-β signaling pathway. The TGF-β signaling pathway is involved in the control of several biologic processes, including cell proliferation, differentiation, migration, and apoptosis, and is one of the most commonly altered pathways in human cancers [39,40•]. TGF-β pathway signals are conveyed through serine/threonine kinase receptors to specific intracellular mediators known as Smad proteins [41]. There are at least eight Smad proteins, divided into three functional classes: 1) receptor-activated Smads (R-Smads)—Smad1, Smad2, Smad3, Smad5, and Smad8; 2) comediator Smads—Smad4 and Smad10; and 3) inhibitory Smads—Smad6 and Smad7. Smad protein activity also is modulated by adaptor proteins such as SARA and β2-spectrin (β2SP) and functional interactions with multiple other signal transduction pathways [42,43]. Downstream targets of TGF-β signaling are key cell cycle checkpoint genes, including CDKN1A (p21), CDKN1B (p27), and CDKN2B (p15), and their activation leads to growth arrest [39].
TGF-β serves as a tumor suppressor in normal intestinal epithelium by inhibiting cell proliferation and inducing apoptosis. Many CRCs escape the tumor-suppressor effects of TGF-β and are resistant to TGF-β–induced growth inhibition (Fig. 1) [44]. In fact, during the late stages of colorectal carcinogenesis, TGF-β acts as a tumor promoter and is highly expressed. Moreover, the TGF-β receptor type II gene (TBR2) contains microsatellite sequences prone to replication errors, especially in the presence of MMR gene inactivation [45]. Frame-shift mutations of TBR2 are found in more than 80% of microsatellite instability–positive CRCs [46]. Mutations in the type I receptor gene (TBR1) also have been identified in human CRC cell lines, and reconstitution of TBR1 expression has been shown to reduce tumorigenicity [47]. Smad4 also is mutated in 16% to 25% of CRC cases, and alterations of Smad2 have been identified in approximately 6% of CRCs [47]. Similarly, mice with a homozygous deletion of Smad3 also have been found to develop an aggressive CRC at an early age, depending on the genetic background of the mice [48].
Interestingly, β2SP, a mediator of Smad3/4 nuclear translocation, correlates with Smad4 expression in human CRC specimens. An analysis of 21 CRC specimens with normal human colon tissue controls demonstrated similar patterns of β2SP and Smad4 expression at the tips and crypts of colonic mucosa. Further analysis demonstrated reduced β2SP in Dukes’ stage B1 tissues. Moreover, mouse genetic studies have found that 3 of 19 6- to 8-month-old β2SP+/−/Smad4+/− mutant mice developed colonic adenomas, compared with none of the wild-type controls or Smad4+/− mutant mice. Studies suggest that β2SP is necessary to confer cell polarity and maintain cell architecture and its loss is associated with the hyperplasia-to-adenoma transition (Fig. 1). Loss of β2SP combined with loss of Smad4 also is seen in advanced and metastatic CRC [49]. These data indicate a strong role for β2SP in TGF-β signaling and in the suppression of early CRC and, later, in metastatic disease with Smad4.
Emerging new data also suggest a role for TGF-β signaling in gut endoderm development and the transition of stem cells to a more differentiated phenotype. The TGF-β signaling pathway appears to be most prominent at the interface between development and cancer in gut epithelial cells [50]. Studies have localized TBR2 to differentiated cells of the villus as well as undifferentiated cells near the bottom of the crypt [51]. Recent studies also suggest that the TGF-β and Wnt pathways synergistically promote CRC tumorigenesis. Compound heterozygous ApcΔ716/+/Smad4+/− mice develop larger colon polyps that can progress to malignant adenocarcinoma [52]. Smad4 heterozygosity also increased tumor multiplicity in the Apc+/N1638 mice [53]. TBR2 inactivation in intestinal epithelial cells of Apc+/N1638 mice also promoted transformation and invasion of tumors initiated by APC mutation in a cell-autonomous manner [54]. The results of these studies confirm that disruption of both TGF-β and Wnt signaling cooperate to drive tumor initiation, likely in colonic stem cells, and progression in vivo.
Therapeutics Targeting CRC Stem Cells
The stem cell model of colon carcinogenesis has important implications for colon cancer prevention and therapy. Elimination or reduction of transformed colonic stem cells likely is the most effective prevention strategy and depends on proper identification of CRC stem cells and the pathways disrupted within them.
Several studies already have targeted stem cells based on their expression of stem cell markers. For example, in acute myeloid leukemia, CD44 has been targeted with an activating monoclonal antibody, H90, resulting in a reversal of the differentiation blockade [55]. Similarly, recent studies demonstrated that the upregulation of IL-4 cytokine in CD133+ CRC stem cells is an important mechanism that protects these tumorigenic cells from apoptosis [56••,57]. These cell isolates were more resistant to standard chemotherapeutic agents, 5-fluorouracil, or oxaliplatin. The ability of these agents to decrease tumorigenic growth, however, was significantly increased when cells were first treated with antibodies to IL-4. This phenomenon was confirmed in xenografts in which the addition of anti–IL-4 antibodies significantly reduced tumor growth after chemotherapy [56••]. More recently, chemoresistant CRC cell lines (HT29/5FU and HT29/O×R) were developed following exposure of the human CRC cell line HT29 to increasing doses of 5-fluorouracil and oxaliplatin. These cell lines demonstrated marked enrichment of CD133+ and CD44+ cells. Phosphorylated and total insulin-like growth factor receptor (IGF-IR) levels also were increased in the resistant cell lines. Tumors derived from the chemoresistant cell lines subsequently exhibited significantly greater growth inhibition than parental cells when exposed to an IGF-IR monoclonal antibody, thereby demonstrating that IGF-IR activation provided for enhanced sensitivity of CRC stem cells [58].
Other potential targets include specific targeting of symmetric stem cell division. Boman et al. [59•] used computer modeling to demonstrate that symmetric stem cell division is responsible for the exponential increase of clonal subpopulations in CRC development. They subsequently hypothesized that symmetrically dividing CRC stem cells may provide an effective target of therapy [40•]. Systemic therapies designed to effectively treat CRC stem cells, however, must act to control or eliminate symmetric cancer stem cell division in tumors while minimally affecting normal stem cell division in nontumor tissues. Therefore, until a clearer understanding of symmetric cell division in tumor and nontumor tissue emerges, these therapies will remain only potentially attractive.
Another attractive approach to CRC stem cell therapy likely involves targeting of disrupted pathways key to stem cell self-renewal in CRC, such as Wnt, Hedgehog, Notch, and TGF-β. Such an approach, however, requires specific identification and delineation of the molecular pathways used by CRC stem cells and identification of agents that can either block the pathways or propel CRC stem cells to differentiate, thereby enhancing their sensitivity to chemotherapeutic agents. One such proposed agent is dietary Ca2+ supplementation. Dietary loading with Ca2+ has been extensively shown to reduce colon cell proliferation, the extent of the crypt’s proliferative zone, and colon carcinogenesis in humans and rodents [34]. Malignant transformation of colon cells, however, leads to decreased sensitivity of these cells to Ca2+ and disruption of their apoptotic mechanisms. In fact, Ca2+ supplementation is reported to stimulate colon cell proliferation in adenomatous polyps. This apparent antagonistic response likely is mediated by disrupted Wnt signaling in colorectal tumors and demonstrates the tightly linked and regulated process of colonic stem cell self-renewal, proliferation, and differentiation [34]. However, targeting of Wnt-driven stem cell proliferation, along with dietary Ca2+ loading to restrain APC and CaSR and promote adherens junctions, may prove an effective strategy and highlights the necessity of novel combinations of therapy targeting the multifaceted nature of CRC stem cells.
A final potential target is the stem cell niche. Several major pathways, including Wnt, Bone Morphogenic, and Notch, also are key to maintaining the stem cell microenvironment [60]. The stem cell niche is essential to modulate the capacity of colonic stem cells to proliferate, migrate, or invade. Therefore, strategies targeting the CRC stem cell niche also are an attractive option and may prove effective as CRC therapy.
Conclusions
The identification of tumor-initiating CRC stem cells has had a significant effect on approaches to CRC prevention and therapy. Novel therapeutics for CRC must now target CRC stem cells. Likely approaches to CRC stem cell therapy include development of agents specifically targeting functional biomarkers or molecules activated in marker-specific populations, agents against disrupted signaling pathways in CRC stem cells, and agents targeting symmetric stem cell division and the stem cell niche. There are many unanswered questions, however, about CRC stem cells, and future research must focus on properly identifying these cells and delineating disrupted pathways within them. The answers to these questions will provide the basis for developing novel therapeutics for advanced and difficult-to-treat CRC.
Acknowledgments
The authors wish to thank Wilma Jogunoori for critical review of the manuscript.
This work is supported by National Institutes of Health grants #PO1 CA130821 (LM), #RO1 CA106614 (LM), and #RO1 CA042857 (LM) and the Ben Orr Award (LM).
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.National Cancer Institute [Accessed July 2009];Colon cancer treatment (PDQ) Available at http://www.cancer.gov/cancertopics/pdq/treatment/colon/healthprofessional/
- 2.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 5.Huang EH, Wicha MS. Colon cancer stem cells: implications for prevention and therapy. Trends Mol Med. 2008;14:503–509. doi: 10.1016/j.molmed.2008.09.005.This is an excellent review of the colon cancer stem cell model and hypotheses of potential targets.
- 6.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 7.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 8.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 9.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 11.Kirkland SC. Clonal origin of columnar, mucous, and endocrine cell lineages in human colorectal epithelium. Cancer. 1988;61:1359–1363. doi: 10.1002/1097-0142(19880401)61:7<1359::aid-cncr2820610714>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura S, Wakabayashi N, Toyoda K, et al. Expression of Musashi-1 in human normal colon crypt cells: a possible stem cell marker of human colon epithelium. Dig Dis Sci. 2003;48:1523–1529. doi: 10.1023/a:1024763723240. [DOI] [PubMed] [Google Scholar]
- 14.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology. 2005;129:1567–1580. doi: 10.1053/j.gastro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.May R, Riehl TE, Hunt C, et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg J, Genander M, Halford MM, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196.This study demonstrates Lgr5, an intestinal Wnt target gene, is a potential colonic stem cell marker.
- 18.van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 19.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 21.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 25.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104.This study demonstrates the potential of CD44 and ESA as colon cancer stem cell markers using fluorescence-activated cell sorting for these enriched cells from tumor specimens. Injecting a few of these cells into NOD/SCID mice reconstituted a phenotypically similar tumor.
- 26.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372.This study enriched CD133+ cells using fluorescence-activated cell sorting from colon cancer specimens. Injecting a few of these cells into NOD/SCID mice reconstituted a phenotypically similar tumor.
- 27.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384.This study also enriched CD133+ cells from colon cancer specimens and demonstrated maintenance of these cells in vitro.
- 28.Ieta K, Tanaka F, Haraguchi N, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol. 2008;15:638–648. doi: 10.1245/s10434-007-9605-3. [DOI] [PubMed] [Google Scholar]
- 29.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401.This study raised doubts about the specificity of CD133 as a marker of colon cancer stem cells. CD133 high- and low-expressing cells generated tumors in NOD/SCID mice.
- 30.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 31.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 32.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 33.Pecina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17. doi: 10.1186/1475-2867-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield JF. Calcium, calcium-sensing receptor and colon cancer. Cancer Lett. 2009;275:9–16. doi: 10.1016/j.canlet.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregorieff A, Pinto D, Begthel H, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Preston SL, Wong WM, Chan AO, et al. Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 2003;63:3819–3825. [PubMed] [Google Scholar]
- 38.Boman BM, Fields JZ, Cavanaugh KL, et al. How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res. 2008;68:3304–3313. doi: 10.1158/0008-5472.CAN-07-2061. [DOI] [PubMed] [Google Scholar]
- 39.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 40.Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828–2838. doi: 10.1200/JCO.2008.17.6941.Excellent review of the colon cancer stem cell model including new markers and its role in the development of colon cancer from hereditary and inflammatory mechanisms.
- 41.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 42.Tsukazaki T, Chiang TA, Davison AF, et al. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Katuri V, Dillner A, et al. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 44.Hoosein NM, McKnight MK, Levine AE, et al. Differential sensitivity of subclasses of human colon carcinoma cell lines to the growth inhibitory effects of transforming growth factor-beta 1. Exp Cell Res. 1989;181:442–453. doi: 10.1016/0014-4827(89)90101-8. [DOI] [PubMed] [Google Scholar]
- 45.Ilyas M, Efstathiou JA, Straub J, et al. Transforming growth factor beta stimulation of colorectal cancer cell lines: type II receptor bypass and changes in adhesion molecule expression. Proc Natl Acad Sci U S A. 1999;96:3087–3091. doi: 10.1073/pnas.96.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayama T, Miyanishi K, Hayashi T, et al. Colorectal cancer: genetics of development and metastasis. J Gastroenterol. 2006;41:185–192. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Pasche B. TGF-beta signaling alterations and susceptibility to colorectal cancer. Hum Mol Genet. 2007;16 Spec No 1:R14–R20. doi: 10.1093/hmg/ddl486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thiagalingam S, Lengauer C, Leach FS, et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 49.Tang Y, Katuri V, Srinivasan R, et al. Transforming growth factor-beta suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Res. 2005;65:4228–4237. doi: 10.1158/0008-5472.CAN-04-4585. [DOI] [PubMed] [Google Scholar]
- 50.Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 51.Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci U S A. 1989;86:1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaku K, Oshima M, Miyoshi H, et al. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 53.Alberici P, Jagmohan-Changur S, De Pater E, et al. Smad4 haploinsufficiency in mouse models for intestinal cancer. Oncogene. 2006;25:1841–1851. doi: 10.1038/sj.onc.1209226. [DOI] [PubMed] [Google Scholar]
- 54.Munoz NM, Upton M, Rojas A, et al. Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res. 2006;66:9837–9844. doi: 10.1158/0008-5472.CAN-06-0890. [DOI] [PubMed] [Google Scholar]
- 55.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 56.Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001.This excellent review demonstrates the key role of IL-4 in tumorigenic growth and its targeting via a specific antibody as a means of decreasing colon cancer tumor progression.
- 57.Francipane MG, Alea MP, Lombardo Y, et al. Crucial role of interleukin-4 in the survival of colon cancer stem cells. Cancer Res. 2008;68:4022–4025. doi: 10.1158/0008-5472.CAN-07-6874. [DOI] [PubMed] [Google Scholar]
- 58.Dallas NA, Xia L, Fan F, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boman BM, Wicha MS, Fields JZ, Runquist OA. Symmetric division of cancer stem cells: a key mechanism in tumor growth that should be targeted in future therapeutic approaches. Clin Pharmacol Ther. 2007;81:893–898. doi: 10.1038/sj.clpt.6100202.This interesting review hypothesizes the specific targeting of symmetric cell division as a means of decreasing the exponential clonal growth of colonic tumors.
- 60.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]