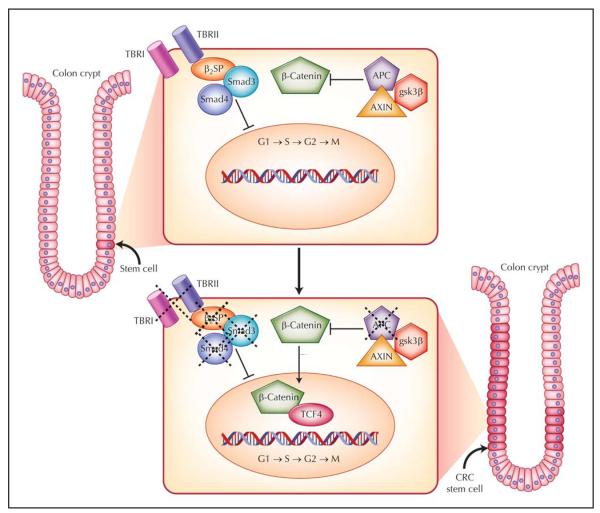
Figure 1.
The colorectal cancer (CRC) stem cell model. In the normal state, stem cells are located near the base of the crypt. Inactive Wnt signaling and activated transforming growth factor (TGF)-β signaling regulate cell growth and mitigate overactivation of cell cycle–related target genes. In hereditary colon cancer syndromes such as familial adenomatous polyposis, the Wnt signaling pathway is activated secondary to a functional abnormality in the adenomatous polyposis coli (APC) protein. The defect in this protein permits β-catenin to translocate to the nucleus to activate downstream transcription factors and growth-related targets. This likely occurs in CRC stem cells, resulting in the generation of many “preneoplastic” colon polyps. Disruption of TGF-β signaling via loss of β2-spectrin (β2SP), Smad4, or the type II receptor (TBRII) synergistically promotes CRC tumorigenesis, likely in stem cells. gsk3β—glycogen synthase kinase 3β; TBRI—type I receptor.