Figure 2.
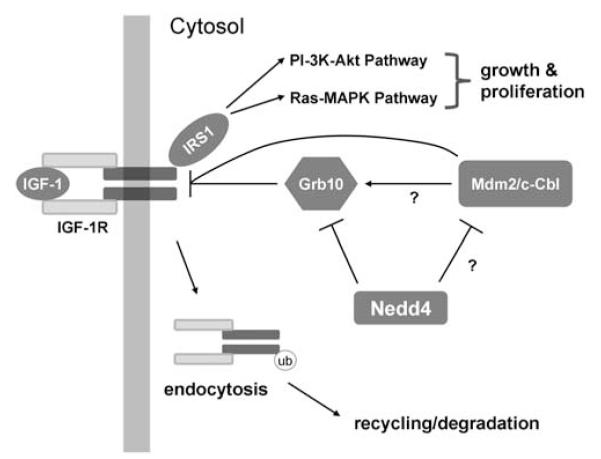
A possible model of Nedd4 function in regulating IGF-1 signaling. Ligand (IGF-1) interaction with IGF-1R results in autophosphorylation of the receptor, which then phosphorylates insulin receptor substrates, such as IRS1. IRS molecules act as docking sites for other proteins, which lead to the activation of both PI3K (phosphoinositide-3 kinase) and MAPK (mitogen-activated protein kinase) signaling pathways, and growth and proliferation of the cells. IGF-1R activity is regulated by ubiquitin-mediated endocytosis, and the endocytosed receptors can be degraded or recycled back to the plasma membrane. Nedd4 might have multiple roles in the regulation of IGF-1R. Nedd4 has been shown to be involved in the downregulation of Grb10, an adaptor protein which binds directly to IGF-1R. Grb10 has been proposed to mediate the interaction between IGF-1R and Nedd4. Other ubiquitin ligases, such as Mdm2 and c-Cbl, have been shown to target IGF-1R for degradation. It is not clear, whether these interactions are mediated by Grb10
