Abstract
Rationale
Patients treated with peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist manifest favorable metabolic profiles associated with increased plasma adiponectin (APN). However, whether increased APN production as a result of PPAR-γ agonist treatment is an epiphenomenon or is causatively related to PPAR-γ’s cardioprotective actions remains completely unknown.
Objective
To determine the role of APN in rosiglitazone (RSG) cardioprotection against ischemic heart injury.
Methods and Results
Adult male wild type (WT) and APN knockdown/knockout (APN+/− and APN−/−) mice were treated with vehicle or rosiglitazone (RSG, 20 mg/kg/day), and subjected to coronary artery ligation 3 days after beginning treatment. In WT mice, RSG (7 days) significantly increased adipocyte APN expression, elevated plasma APN levels (2.6-fold), reduced infarct size (17% reduction), decreased apoptosis (0.23±0.02% vs. 0.47±0.04% TUNEL positive in remote non-ischemia area), attenuated oxidative stress (48.5% reduction), and improved cardiac function (P<0.01). RSG-induced APN production and cardioprotection were significantly blunted (P<0.05 vs. WT) in APN+/−, and completely lost in APN−/− (P>0.05 vs. vehicle-treated APN−/− mice). Moreover, treatment with RSG for up to 14 days significantly improved the post-ischemic survival rate of WT mice (P<0.05 vs. vehicle group), but not APN knockdown/knockout mice.
Conclusion
PPAR-γ agonists’ cardioprotective effects are critically dependent on its APN stimulatory action, suggesting that under pathologic conditions where APN expression is impaired (such as advanced type-2 diabetes), the harmful cardiovascular effects of PPAR-γ agonists may outweigh its cardioprotective benefits.
Keywords: Diabetes, Myocardial infarction, Adipocytokine, Signaling
Diabetes mellitus and its resultant cardiovascular disease are a dominant public health problem. The hyperglycemia and hyperlipidemia associated with diabetes mellitus not only result in vascular injury causing greater myocardial infarction (MI) mortality, but also directly impact ischemic cardiomyocytes adversely, causing larger infarct size1,2 and more severe heart failure3 after MI, further contributing to higher mortality. Effective therapeutic interventions enhancing tissue insulin sensitivity and improving cellular metabolism may therefore not only attenuate diabetic injury, but also reduce the risk of myocardial infarction and death of cardiovascular etiology.
Peroxisome proliferator-activated receptors (PPARs, including PPARα, −δ and –γ) are transcription factors belonging to the nuclear receptor super family4. PPARγ agonists such as rosiglitazone (RSG) and pioglitazone are insulin sensitizers, and have been used widely in the treatment of type-2 diabetes. Numerous experimental studies have demonstrated that PPARγ agonist treatment not only attenuates vascular injury in diabetic animals5–7, thus reducing the incidence of MI, but also exerts direct protective effect on ischemic cardiomyocytes8,9 and improves post-MI cardiac function recovery. Moreover, several clinical studies10–12, including one recently published13, have demonstrated that RSG treatment reduces coronary events following percutaneous coronary intervention and retards atherosclerosis disease progression. However, two meta-analyses14,15 reported that long-term treatment of advanced diabetic patients with rosiglitazone was associated with a significantly increased risk of myocardial infarction and death from cardiovascular causes. Furthermore, a recent study has demonstrated that elderly diabetic patients treated with RSG are at higher risk of developing heart failure16 and mortality. These results raised a major concern for utilizing RSG in diabetic patients17. The etiology of the sizable discrepancy between experimental and clinical studies, as well as between different clinical trails, remains completely unknown.
Adiponectin (APN), an adipocytokine secreted from adipose tissue18,19, is normally present in plasma at concentrations up to 30 µg/ml. It is markedly down-regulated in association with obesity-linked diseases, including coronary artery disease and type-2 diabetes20. Clinical observations have revealed that total plasma APN concentrations are inversely correlated with the risk of coronary artery disease21 and myocardial infarction22. Treatment with PPARγ agonists stimulates APN production in adipocytes, and APN is the mediator through which PPARγ agonists achieve their insulin sensitization and metabolic benefits23. Moreover, recent experimental studies have demonstrated that APN plays an essential role in both the hepatic insulin sensitization and the plasminogen-activator-inhibitor-1 production suppressive effect of PPARγ agonists24,25. However, whether increased APN production as a result of PPARγ agonist treatment is solely an epiphenomenon, or is causatively related to PPAR-γ’s cardioprotective actions remains completely unknown.
Therefore, the aims of the present study were to determine the role of APN in RSG cardioprotection, and to investigate how reduced APN production (as seen in both advanced and elderly diabetic patients) may alter the cardiovascular actions of RSG.
Materials and Methods
Adult male adiponectin knockdown (APN+/−) and knockout (APN−/−) mice, and their male littermate controls (WT) were used in the present study. Generation, breeding, phenotype characteristics, and genotyping of these mice have previously been described in detail26. All experiments were performed in adherence with the National Institutes of Health Guidelines on the use of Laboratory Animals, and were approved by the Thomas Jefferson University Committee on Animal Care.
Experimental protocols
On day zero of the experiment, mice were randomized to receive vehicle (control), rosiglitazone (RSG, 20 mg/kg/day25, oral gavage, Cayman Chemical, Ann Arbor, Michigan), MnTE-2-PyP5+ (a cell permeable SOD mimic, 10 mg/kg/day, IP27) or RSG+MnTE-2-PyP5+ (to determine the possible involvement of superoxide in RSG cardiac signaling). Seventy two hours after the first drug administration, mice were anesthetized with 2% isoflurane, and myocardial ischemia (MI) was produced by temporarily exteriorizing the heart via a left thoracic incision, and ligation of the left anterior descending (LAD) coronary artery with a 6-0 silk suture. Sham-operated control mice (sham MI/R) underwent the same surgical procedures, except that the suture placed under the left coronary artery was not tied. After 4 days (7 total days of RSG treatment) of MI, animals were re-anesthetized, aortic blood (0.5 ml) was collected, and the epididymal fat pad and heart were removed. Adipocyte APN mRNA expression, plasma APN level, myocardial infarct size, myocardial apoptosis, and superoxide production in non-ischemic regions were determined according to the below described procedures. To determine the effect of RSG on survival rate, WT, APN+/− or APN−/− mice were continuously treated with RSG for up to 14 days, or until animal death.
Determination of adipocyte APN mRNA expression and plasma APN concentration
Total RNA was isolated from white adipose tissue (epididymal fat pad) using TRIzol reagent (Invitrogen), and cDNA was synthesized from 1 µg RNA, with the iScript cDNA Synthesis kit (BioRad). Adiponectin mRNA levels was quantified by real-time PCR with the use of SYBR Green (Applied Biosystems, Foster City, Calif), and corrected for GAPDH mRNA level. Total plasma adiponectin concentration was determined with a mouse adiponectin ELISA kit (Phoenix Pharmaceuticals, Inc., Belmont, CA) per manufacturer’s instructions.
Determination of myocardial apoptosis and myocardial infarct size
Myocardial apoptosis was determined by TUNEL staining and caspase-3 activity as described previously28. Myocardial infarct size was determined by Evans blue-TTC double staining methods28, and expressed as a percentage of infarct area over ischemic area (area-at-risk, AAR).
Determination of cardiac function
Cardiac function was determined by echocardiography (VisualSonics VeVo 770 imaging system) 4 days after coronary occlusion (7 days after RSG treatment), and by left ventricular (LV) catheterization (1.2-Fr micromanometer, Millar Instruments, Houston, TX) prior to animal sacrifice (11 days after coronary occlusion/14 days after RSG treatment). Both methods have been described in detail in our previous publications28, 29.
Quantification of superoxide production in cardiac tissue
Myocardial superoxide content (non-ischemic area) was quantified by lucigenin enhanced luminescence and the cellular origin of reactive oxygen species was determined by dihydroethidium staining (DHE, Molecular Probes, Carlsbad, CA) as described previously29.
Statistical analysis
All values in the text and figures are presented as means±SEM of n independent experiments. All data (except survival and Western blot density) were subjected to 2-way ANOVA followed by Bonferoni correction for post-hoc t test. Animal survival was evaluated by the Kaplan-Meier method, and the log-rank test was used to compare survival curves between vehicle-treated and RSG-treated groups. Western blot densities were analyzed with the Kruskal-Wallis test, followed by Dunn’s post-hoc test. Probabilities of 0.05 or less were considered to be statistically significant. The authors had full access to, and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written.
Results
Adipocyte APN expression, plasma APN levels, and plasma TNFαlevels
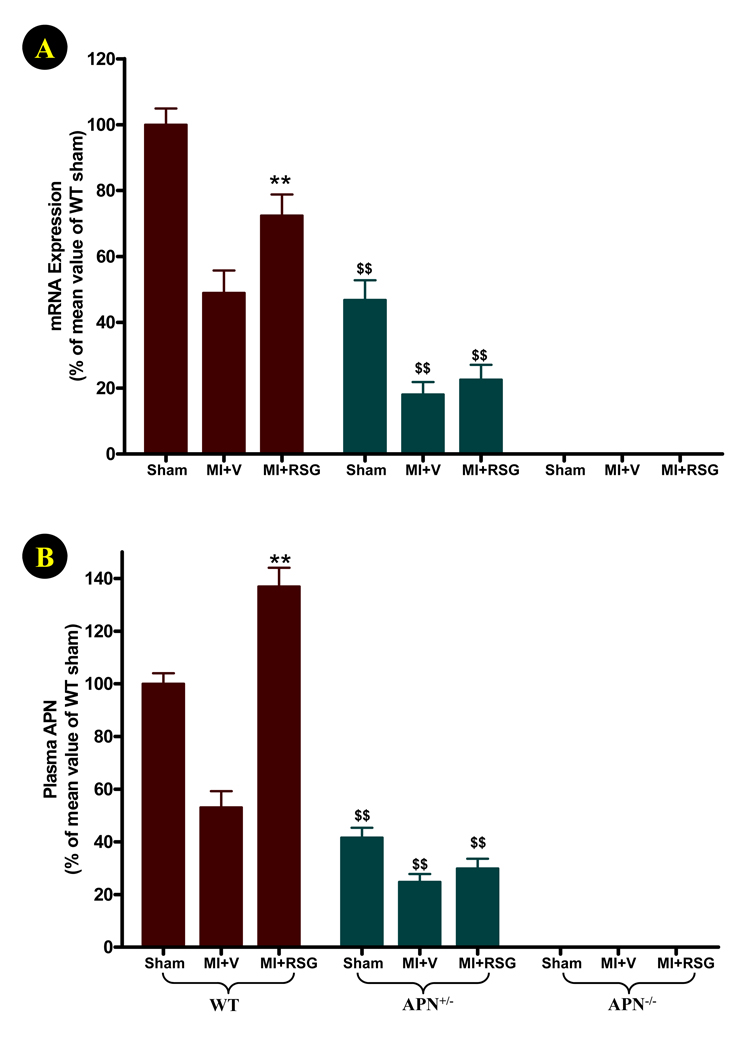
In WT animals, high levels of adipocyte APN mRNA expression and plasma APN (8.4±1.9 µg/ml) were detected. As expected, both adipocyte APN expression and plasma APN levels were markedly reduced in APN+/− animals and undetected in APN−/− animals (Figure 1). In WT animals, MI caused a remarkable reduction in adipocyte APN mRNA expression (47.6±6.8% of sham MI control, P<0.01), and significantly reduced plasma APN levels (53.3±6.1% of sham MI control, P<0.01). In APN+/− mice, both adipocyte APN expression and plasma APN levels were further significantly reduced after MI (Figure 1). Treatment of WT animals with RSG significantly attenuated the inhibitory effect of MI on adipocyte APN mRNA expression (72.4±6.4% of sham MI control, P<0.05 vs. MI+vehicle), and increased plasma APN to a level (136.9±7.1% of sham MI control, P<0.01) that were even higher than mice subjected to sham MI without RSG treatment (P<0.05). Interestingly, the APN stimulatory effect of RSG was almost completely abolished in APN+/− mice. No statistically significant change in either adipocyte APN expression or plasma APN levels were observed in APN+/− mice treated with RSG (Figure 1).
Figure 1.
Adipocyte APN mRNA expression and plasma APN concentrations 4 days after permanent LAD occlusion (1 week after RSG treatment). N=14 to 16 animals/group. **P<0.01 vs. vehicle-treated group; $$P<0.01 vs. WT mice in the same treatment group.
Previous studies have demonstrated that there exists a reciprocal inhibitory relationship between APN and TNFα30. To determine whether TNFα concentration was elevated in APN+/− mice, and thereby potentially counteractive of the RSG APN stimulatory effect, serum TNFα levels were determined by ELISA. There was no significant difference in basal serum TNFα levels between WT, APN+/− and APN−/− mice (7.6±0.81, 7.4±1.1 and 9.1±1.0 pg/ml). However, MI-induced TNFα overproduction was significantly potentiated in APN deficient mice (WT: 28.4±2.1 pg/ml; APN+/−: 86.1±4.3 pg/ml, P<0.01 vs. WT; APN−/−: 138±5.6 pg/ml, P<0.01 vs. WT and APN+/− groups).
Loss of RSG acute cardioprotection in APN knockout mice
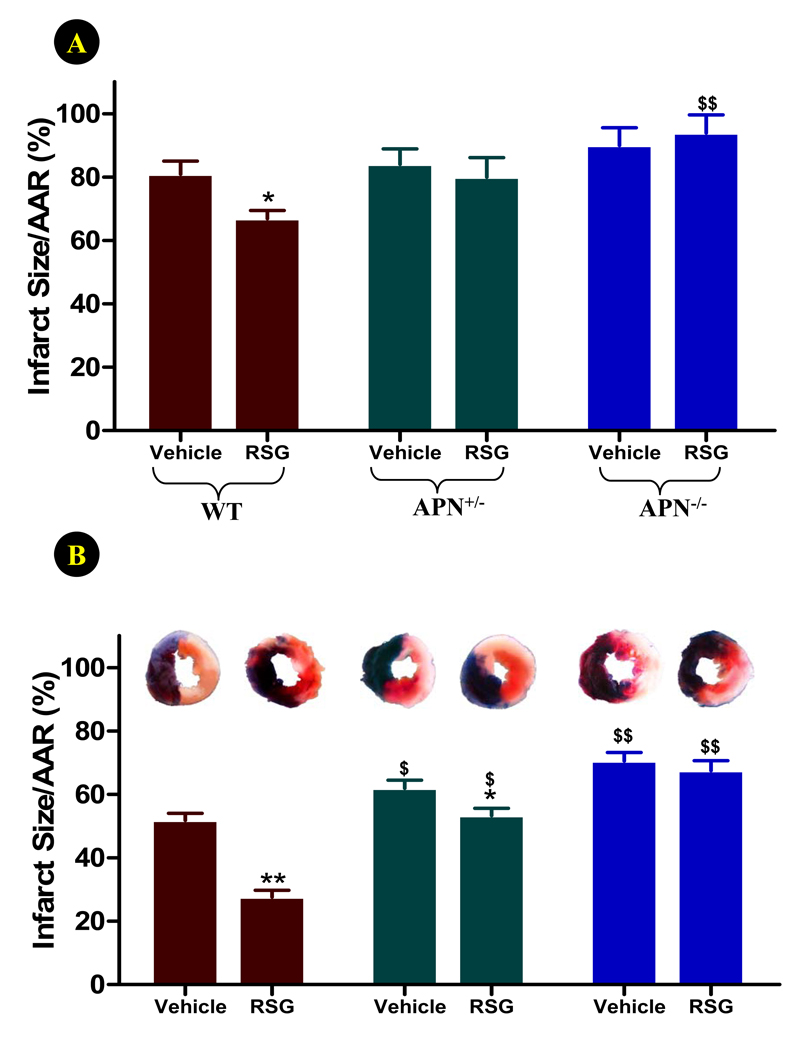
4-days of permanent coronary occlusion caused over 80% cell death in the ischemic area (Figure 2). Treatment of WT mice with RSG modestly reduced myocardial infarct size in this severe, permanent myocardial ischemic model (Figure 2A). In APN knockout mice (both APN+/− and APN−/− mice), infarct size was slightly increased compared to WT animals. However, this difference was not statistically significant. This result is consistent with a recent report demonstrating that permanent coronary occlusion for 4 weeks resulted in comparable myocardial infarct size in WT and APN−/− mice31. More importantly, treatment with RSG failed to reduce infarct size in APN+/− and APN−/− mice, and a very significant difference was observed between RSG-treated WT and APN−/− mice (Figure 2A).
Figure 2.
Effect of RSG treatment on myocardial infarct size (expressed as % of area-at-risk, AAR) 4 days after permanent LAD occlusion (A) or 4 days after reperfusion (30 min ischemia) (B). N=14 to 16 mice/group. *<0.05 vs. vehicle-treated group; $P<0.05, $$P<0.01 vs. WT animals in the same treatment group.
In contrast to the abovementioned results demonstrating no significant infarct size difference between APN−/− and WT mice subjected to permanent ischemia, previous studies by Shibata et al30 and our group29 have demonstrated significantly larger infarct size during temporary ischemia followed by reperfusion in APN−/− than WT mice. Additional experiments were performed to further determine whether APN is also required for RSG cardioprotection in the ischemia/reperfusion model. As summarized in Figure 2B, 30 minutes of ischemia followed by 4 days of reperfusion caused approximately 50% cell death in WT mice. Infarct size was significantly larger in APN+/− (P<0.05) and APN−/− (P<0.01) mice. Moreover, treatment with RSG (in an identical manner as described for the permanent coronary occlusion model) caused a 46% infarct size reduction in WT mice (P<0.01). The protective effect of RSG was significantly attenuated in APN+/− (P<0.05 vs. WT+RSG) and completely lost in APN−/− (P<0.01 vs. WT+RSG) mice.
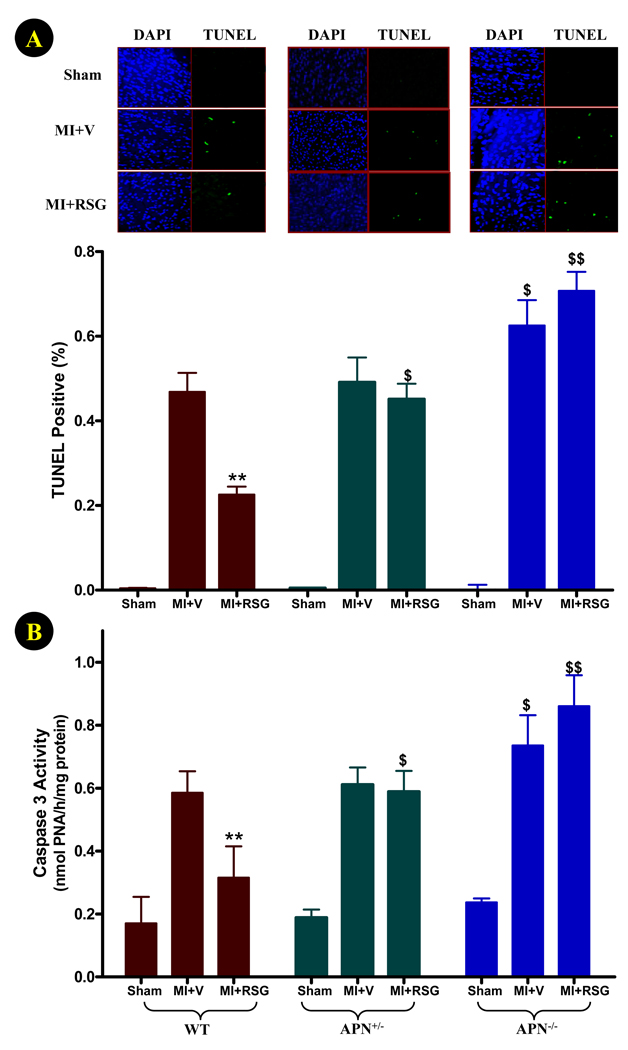
Apoptotic cell death in remote, non-ischemic areas of the heart plays a critical role in post-MI left ventricular remodeling, and greatly influences cardiac function deterioration. We thus determined whether RSG may attenuate apoptotic cell death in non-ischemic cardiac regions in the permanent ischemic model, and more importantly, whether APN is requisite for the anti-apoptotic effects of RSG. As illustrated in Figure 3, no TUNEL positive cells were detected in hearts subjected to sham MI, and caspase-3 activity was very low. In WT mice, 4-days of permanent coronary occlusion caused 0.47±0.04% cells in non-ischemic regions to become TUNEL positive, and a 3.4-fold increase of caspase-3 activity (P<0.01). RSG treatment significantly reduced apoptosis, as determined by TUNEL staining and caspase-3 activity (Figure 3). Although similar levels of TUNEL positive staining and caspase-3 activation were observed in APN+/− mice, RSG treatment failed to attenuate cardiomyocyte apoptosis in these animals. In APN−/− mice, permanent coronary occlusion caused greater apoptosis in non-ischemic areas, as evidenced by more TUNEL positive staining (0.63±0.05%, P<0.05 vs. WT) and higher caspase-3 activity (0.74±0.09 nmol PNA/h/mg protein, P<0.05 vs. WT). Moreover, there is a trend of increasing TUNEL index and caspase-3 activity in the non-ischemic area in RSG treated APN−/− mice, although the difference was not statistically significant. The difference in apoptotic cell death between RSG-treated WT and APN−/− mice was highly significant (P<0.01).
Figure 3.
Effect of RSG treatment on myocardial apoptosis (remote, non-ischemic region) determined by TUNEL staining (A) and caspase-3 activity (B) 4 days after permanent LAD occlusion. For TUNEL staining: N=6–8 mice/group; for caspase-3 activity assay: N=14 to 16 mice/group. **<0.01 vs. vehicle-treated group; $P<0.05, $$P<0.01 vs. WT animals in the same treatment group.
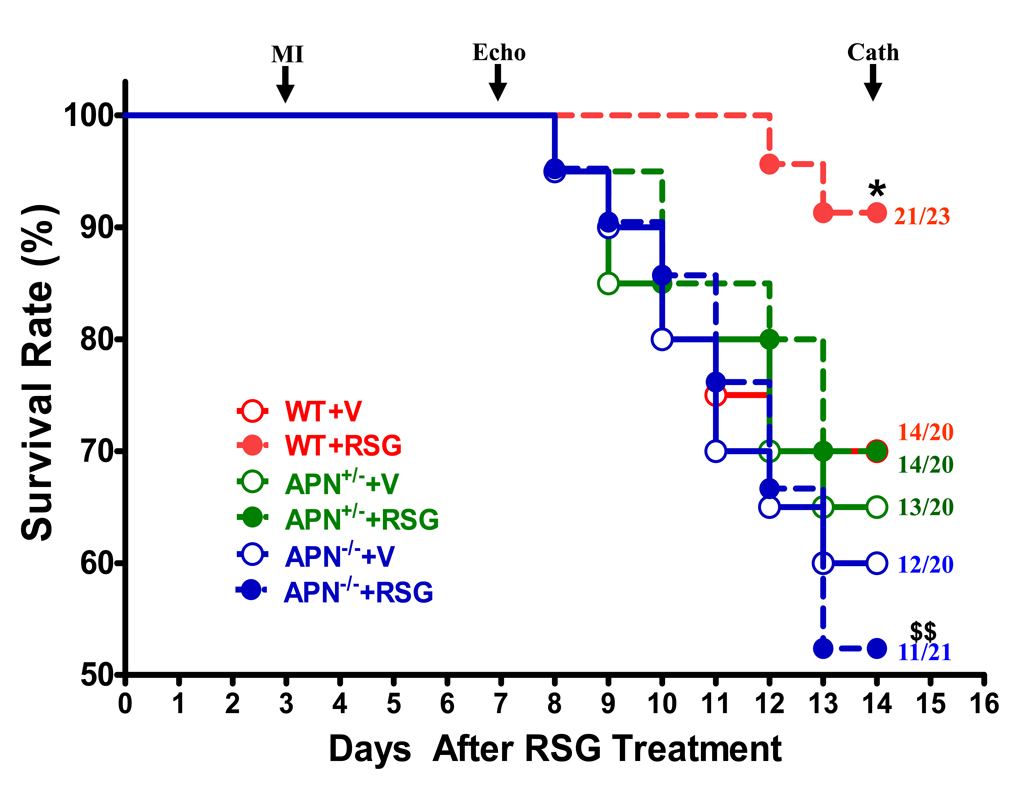
Chronic RSG treatment improved post-MI survival rate in WT, but not in APN knockout mice
Having demonstrated that the acute anti-apoptotic and infarct-sparing effects of RSG were critically dependent upon its APN stimulatory effects, we extended RSG administration for another 7 days, and determined whether APN is required in RSG’s ultimate cardioprotective effects after MI. As summarized in Figure 4, approximately 35% of WT animals receiving vehicle died 11 days after MI. No significant difference in survival rate was observed between WT and APN+/− or between WT and APN−/− mice when treated with vehicle. RSG treatment significantly increased survival rate in WT (91.3% vs. 70.0%, P<0.05) but had no significant effect in APN+/− and APN−/− mice. The survival rate difference between RSG-treated WT and APN−/− mice was highly significant (P<0.01).
Figure 4.
Post-MI survival rate in WT and APN knockout mice treated with vehicle (V) or rosiglitazone (RSG). LAD was permanently occluded 3 days after vehicle or RSG treatment, and drug administration was continued for 14 days (11 days after MI) or until animal death. Numbers at the end of each survival curve represent total animals studied (bottom) and number of animals survived (top). *P<0.05 vs. vehicle-treated WT group; $$P<0.01 vs. RSG-treated WT group.
Treatment with RSG improved cardiac function in WT mice but not in APN knockout mice
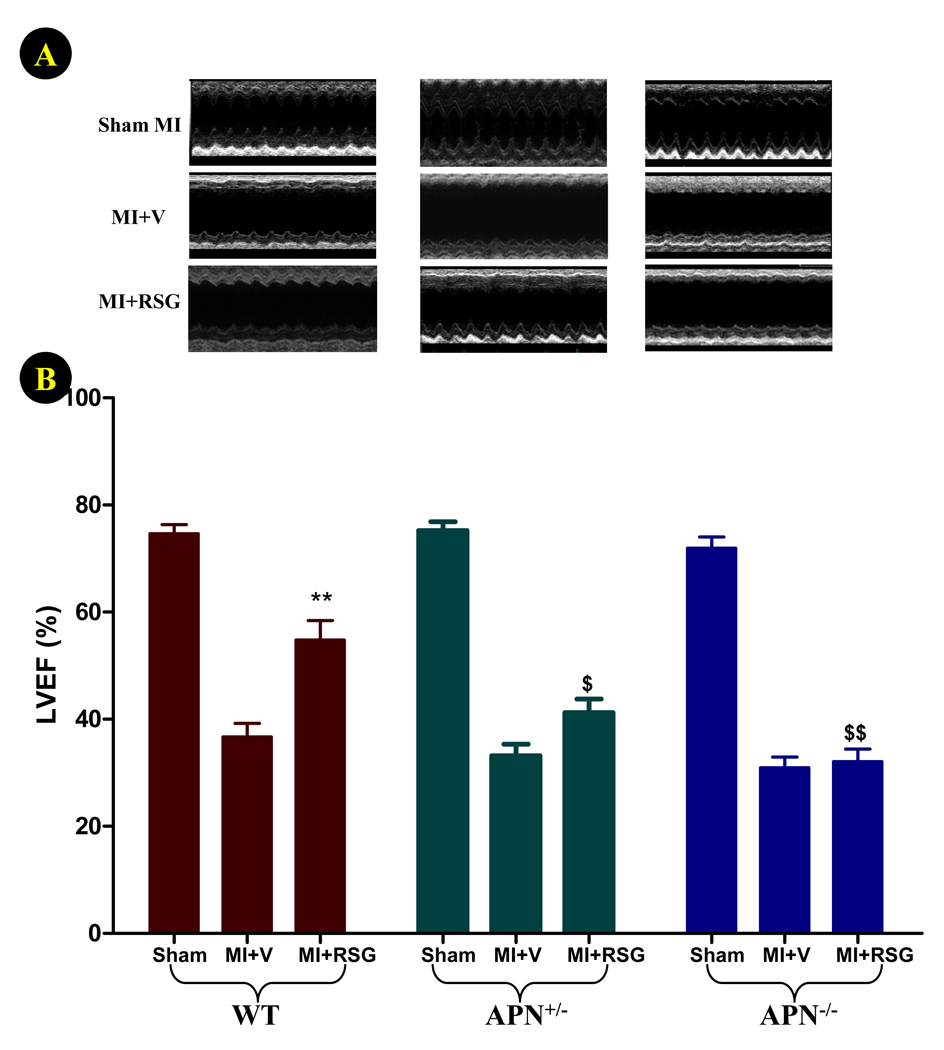
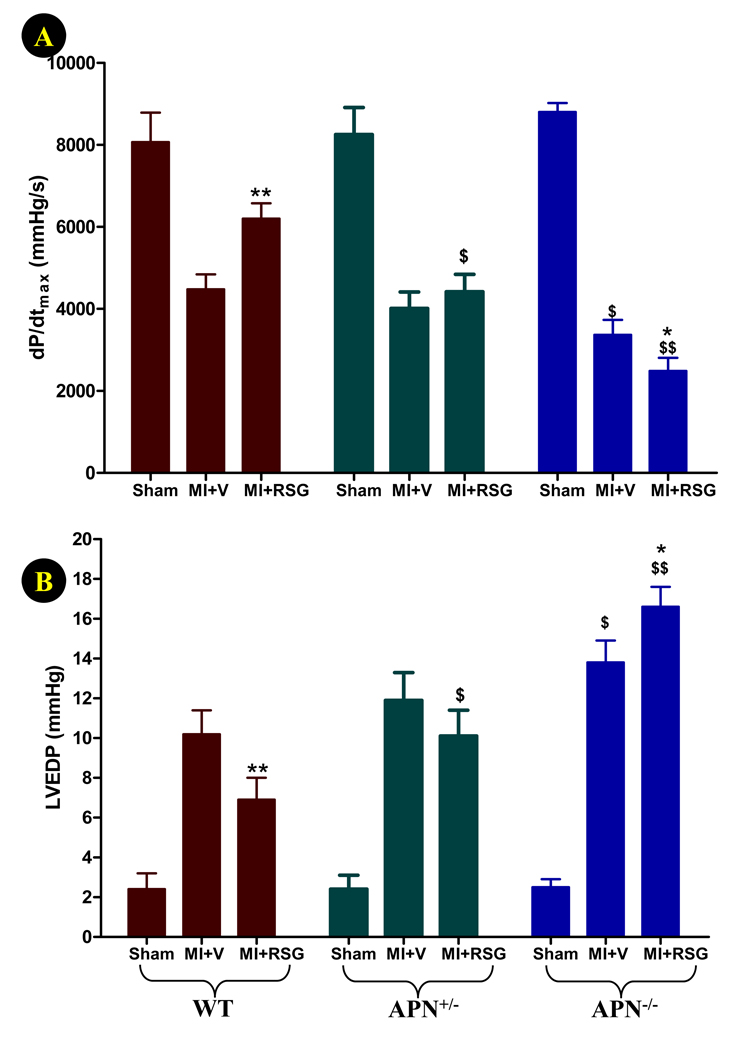
4-days of permanent coronary occlusion in WT and APN knockout mice caused severe cardiac dysfunction (>50% reduction in left ventricular ejection fraction, Figure 5). Although comparable reduction in LVEF was observed in both vehicle-treated WT and APN knockout mice at this relatively early time point, the cardiac response to RSG progressively diminished as APN production decreased. Specifically, RSG significantly increased LVEF in WT mice (P<0.01 vs. vehicle group, Figure 5), slightly but insignificantly improved LVEF in APN+/− mice, and became completely ineffective in APN−/− mice. When MI and RSG treatment were extended for another 7 days, the diverse effect of RSG on cardiac function became more evident. As summarized in Figure 6, dP/dtmax was markedly reduced, and LVEDP was significantly increased in all three groups of MI animals treated with vehicle. Significantly worse cardiac function was observed in APN−/− mice when compared with WT mice at this late time point (Figure 6). Treatment with RSG significantly increased dP/dtmax and reduced LVEDP in WT mice but not in APN+/− mice. Most surprisingly, 14 days of RSG treatment of APN−/− mice further worsened cardiac function, evidenced by lower dP/dtmax and higher LVEDP (Figure 6). The difference between RSG-treated WT and APN−/− mice in all cardiac functional measurements (LVEF, dp/dtmax and LVEDP) was highly significant (P<0.01).
Figure 5.
Effect of RSG treatment on cardiac function determined by echocardiography (A: representative echo recordings; B: left ventricle ejection fraction, LVEF) 4 days after permanent LAD occlusion. N=20 to 23 mice/group. **<0.01 vs. vehicle-treated group; $P<0.05, $$P<0.01 vs. WT animals in the same treatment group.
Figure 6.
Effect of RSG treatment on cardiac function determined by Millar Mikro-Tip® pressure transducer catheter 11 days after permanent LAD occlusion (A: dP/dtmax; B: left ventricular end diastolic pressure, LVEDP). N=11 to 21 mice/group. *P<0.01, **<0.01 vs. respective vehicle group; $P<0.05, $$P<0.01 vs. WT animals in the same treatment group.
Treatment with RSG reduced oxidative stress in WT mice but further enhanced superoxide production in APN−/− mice
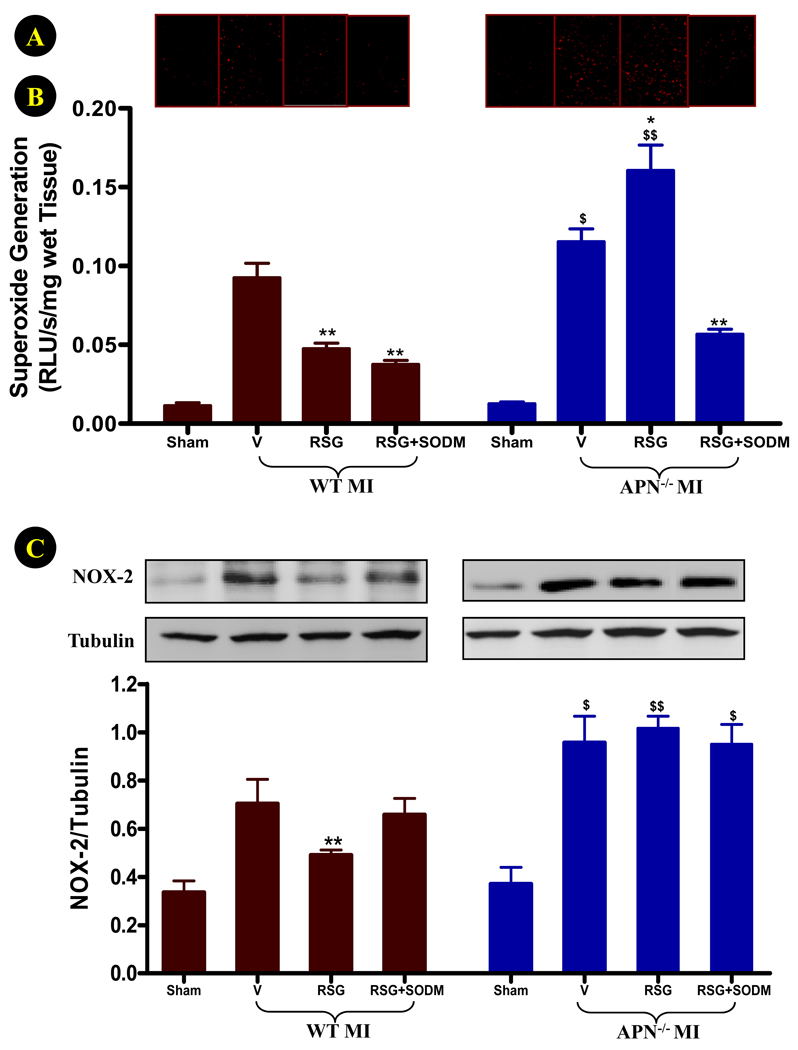
The aforementioned experimental results demonstrated that partial APN loss attenuated, and complete APN absence abrogated, RSG cardioprotection against ischemic heart injury. However, it is unlikely that RSG cardioprotective mechanisms reside exclusively in the APN signaling pathway. A more likely explanation for these results might be that RSG has both harmful and protective effects on the ischemic heart, and a balance of these factors determines the final outcome. In a recent study32, it has been reported that the effect of RSG on myocardial ischemia/reperfusion injury depends on the dose and timing of administration, and that the deleterious effect of high dose RSG administration before ischemia is reversed by an antioxidant (N-acetylcysteine). This result suggests that RSG may enhance oxidative stress in the ischemic heart. To directly examine this possibility, the effect of RSG treatment upon cardiac oxidative stress in WT and APN−/− mice was observed. RSG had no effect on basal superoxide production in sham-operated animals (data not shown). As summarized in Figure 7A, superoxide production (assessed by DHE staining and lucigenin enhanced luminescence) in remote, non-ischemic cardiomyocytes of vehicle-treated APN−/− mice was significantly greater than that of vehicle-treated WT mice. More importantly, treatment with RSG exerted divergent and opposite effects on superoxide overproduction in WT (significantly reduced) and APN−/− (further increased) mice.
Figure 7.
Effect of RSG treatment on superoxide production (A: representative DHE staining sections; B: lucigenin enhanced luminescence assay) and NOX-2 expression (C) in remote, nonischemic region 4 days after permanent LAD occlusion. N=14 to 16 mice/group. *P<0.05, **<0.01 vs. respective vehicle group; $P<0.05, $$P<0.01 vs. WT animals in the same treatment group.
Although ischemia stimulates superoxide production from multiple sources including mitochondria and the xanthine/xanthine oxidase system, NOX-2(gp91phox)-containing NADPH oxidase has been increasingly recognized as the most significant molecule responsible for superoxide overproduction in cardiomyocytes cells33. As summarized in Figure 7B, ischemia-induced NOX-2 overexpression was further increased in APN−/− mice, and the inhibitory effect of RSG on NOX-2 overexpression was completely lost in these animals.
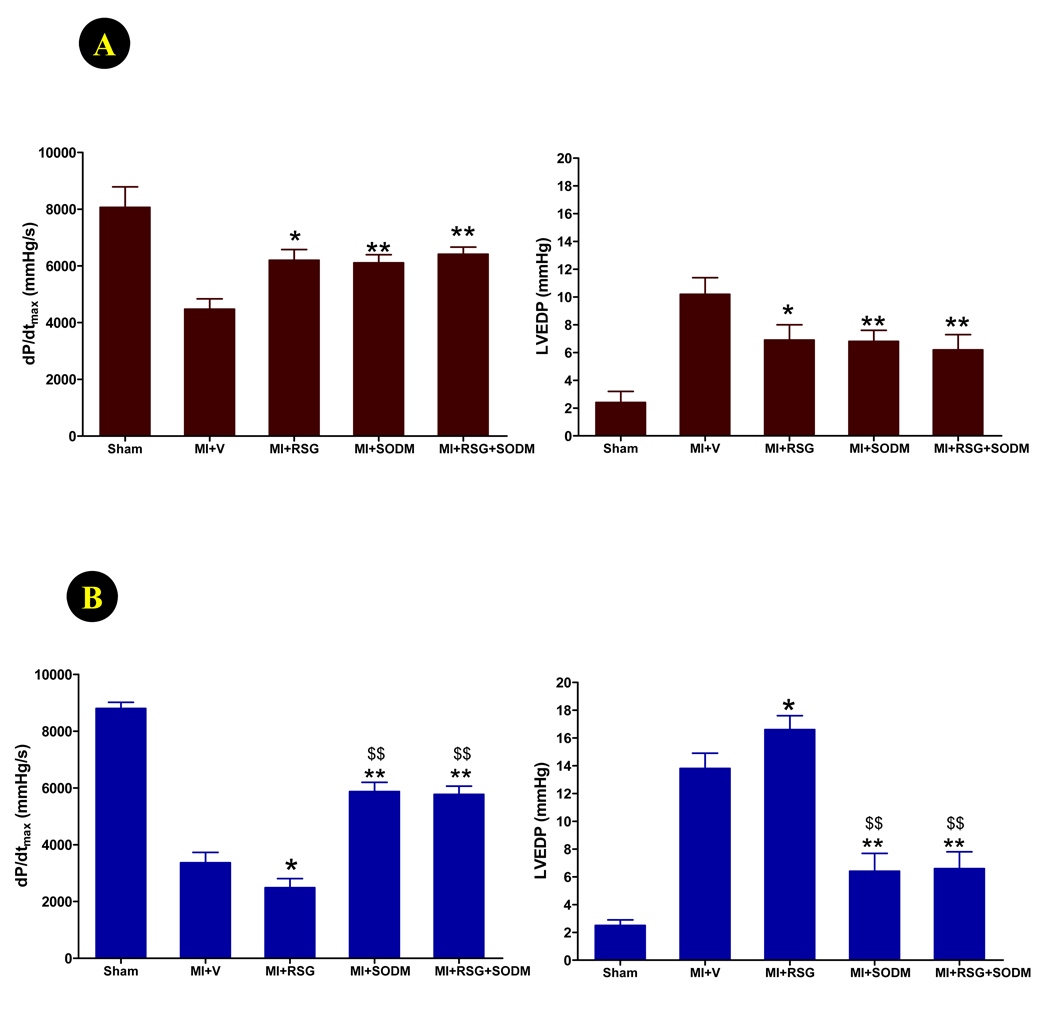
Treatment with a superoxide dismutase mimic (SODM) preferentially improved cardiac function in APN−/− mice vs. WT mice and reversed the detrimental effect of RSG on cardiac function in APN−/− mice
Experimental results summarized in Figure 6 and Figure 7 suggest that, in APN knockout mice, increased oxidative stress may contribute to the detrimental cardiovascular actions of RSG. To obtain more supporting evidence of this hypothesis, we determined whether treatment with a cell membrane permeable superoxide dismutase mimic (SODM), MnTE-2-PyP5+, might reverse or attenuate the adverse effects of RSG on APN−/− mice cardiac function. WT or APN−/− mice were subjected to MI, and treated with vehicle, RSG, SODM, or RSG+SODM for 14 days. As summarized in Figure 8, daily administration of SODM significantly improved cardiac function in WT, as well as in APN−/− mice, but a preferential effect was observed. Specifically, treatment of WT mice with SODM caused a 36.5% increase in dP/dtmax and a 33% reduction in LVEDP (Figure 8A). However, treatment of APN−/− mice with SODM caused a 74.6% increase in dP/dtmax and a 53.6% reduction in LVEDP (Figure 8B). Furthermore, co-treatment of SODM with RSG completely reversed the detrimental effect of RSG on cardiac function in APN−/− mice.
Figure 8.
Effect of RSG, SODM, and their combination on cardiac function determined by Millar Mikro-Tip® pressure transducer catheter 11 days after permanent LAD occlusion in WT (A) and APN−/− mice (B). *P<0.05, **<0.01 vs. MI+vehicle; $$P<0.01 vs. MI+RSG.
APN-KO mice displayed reduced myocardial capillary density, and lost response to RSG
In a permanent myocardial ischemic model, APN has been shown to improve cardiac function by protecting against MI-induced capillary loss in the border zone31. To determine whether RSG-stimulated APN may protect heart by a similar mechanism, capillary density was assessed by CD31 staining of tissue sections in the peri-infarct zone, as described by Shibata et al31. No difference in capillary density was observed in sham-operated WT mice and APN−/− mice (2,769±125 and 2,718±149 capillaries/mm2). Consistent with the reports of Shibata et al, capillary density in peri-infarct zone was significantly lower in APN−/− mice than WT mice (2,016±133 and 2,418±118 capillaries/mm2, P<0.05). Treatment of WT with RSG significantly attenuated MI-induced capillary loss (2,721±109 capillaries/mm2, P<0.05 vs. vehicle). This is similar to a recent study demonstrating that pioglitazone restores ischemia-induced angiogenesis34. However, RSG failed to restores ischemia-induced capillary loss in APN−/− mice (2,138±110 capillaries/mm2).
Discussion
We have made several important observations in the current study. First, we have demonstrated that MI caused significant reduction in APN mRNA expression and plasma APN concentration. RSG treatment of WT mice, but not APN+/−mice, successfully recovered adipocyte APN mRNA expression, and increased plasma APN. Our findings are consistent with previous clinical and experimental model data, reporting plasma APN reduction following MI35, and RSG stimulation of APN production23. However, to our knowledge, our current study is the first reporting RSG treatment efficacy in reversing MI-induced APN inhibition. This result revealed a novel molecular mechanism that may contribute to RSG’s previously reported anti-ischemic and cardioprotective effects9. Interestingly, treatment with RSG increased plasma APN to a level not only significantly greater than that of vehicle-treated MI animals, but also higher than that seen in sham MI animals. The greater effect of RSG on plasma APN level than its effect on adipocyte mRNA expression indicates that RSG may regulate APN protein production at multiple levels, including traditional transcriptional regulation16, and recently reported post-transcriptional regulation36.
Second, we have demonstrated that, even in a model of very severe permanent coronary artery occlusion, RSG treatment was capable of modestly reducing infarct size, significantly decreasing apoptotic cell death in remote, non-ischemic heart regions, and improving cardiac function in WT mice. However, in APN knockout mice, the infarct sparing effect of RSG was completely lost, and slightly higher apoptotic cell death and worse cardiac function were observed. These results further emphasize APN as an indispensable molecule playing a crucial role in RSG’s anti-ischemic, anti-apoptotic, and cardioprotective effects. Substantial evidence now exists that apoptosis is a major player in post-MI cardiac remodeling37. Our current experimental results demonstrated that RSG modestly reduced infarct size but significantly reduced apoptosis in non-ischemic regions. These results suggest that RSG, via its APN stimulatory effects and anti-apoptotic effect, may have therapeutic application in reducing cardiac remodeling and improving cardiac function in MI patients.
Third, we have demonstrated that RSG influence on post-MI survival rate (the ultimate measurement of cardioprotection) is related to APN production. Specifically, RSG treatment significantly improved post-MI survival rate in WT mice, whereas the identical treatment failed to improve the final outcome of MI in APN-deficient mice. Although these results do not assert APN stimulation is the only cardioprotective pathway through which RSG achieves its cardiovascular benefits, it is safe to conclude that APN is indispensable in the overall cardioprotective function of RSG. Moreover, our results suggest that RSG has diverse effects on the cardiovascular system, and its beneficial effects are predominantly achieved through stimulation of APN production. In the absence of APN, the balance between RSG’s protective and detrimental actions is tilted, and the remaining cardioprotective forces of RSG are not strong enough to overcome its cardiac-detrimental side effects. Given that plasma APN is markedly reduced in advanced type-2 diabetic patients20, it is possible that adipocytes may have lost their response to RSG as observed in APN+/− mice in the present study. Under such conditions, the adverse side effects of RSG would predominate unchecked, with the risk of unfavorable cardiovascular outcomes. Although it is impossible to directly extrapolate experimental findings to clinical patients, our current experimental results offer a possible explanation for seemingly divergent results from experimental and clinical RSG studies, as well as between different clinical trails.
Finally, we have demonstrated that treatment with RSG exerted significant anti-oxidant effects in WT mice. This is consistent with a recent report showing that RSG improves myocardial diastolic function in type-2 diabetic patients through oxidative stress reduction38. However, we have demonstrated for the first time that treatment of APN-deficient animals with RSG significantly increased superoxide production, and that co-administration of a cell membrane permeable SOD mimic completely reversed unfavorable effect of chronic RSG treatment on cardiac function. These results indicate that the anti-oxidant effect of RSG reported in previous studies39,40 is achieved through APN, and that the unfavorable action of RSG on post-ischemic oxidative stress is likely responsible for its cardiodepressive effects observed when APN production is diminished.
In summary, our current study demonstrated that APN is an indispensable molecule in RSG’s anti-oxidative, anti-ischemic, anti-apoptotic, a n d cardioprotective actions. Under pathologic conditions where plasma APN is markedly reduced and/or APN stimulatory action of RSG is impaired, RSG treatment may upregulate superoxide production, culminating in unfavorable cardiovascular outcomes. These results create an impetus to identify alternative treatments, such as combining RSG with antioxidant agents, in a fashion potentially circumventing the adverse PPARγ agonist side effects, achieving maximal cardiovascular benefits in both advanced and elderly type-2 diabetic patients requiring extended PPARγ agonist treatment.
Limitations
Greater than 90% of animals died when no laboratory personnel were present, and we were unable to precisely determine the cause of death. Additional studies with a continuous animal monitoring system are required. In addition, the signaling mechanism responsible for RSG enhancement of superoxide production in the APN−/− animals remains unclear. Possible involvement of COX-230 and other cardioprotective signaling molecules41, specifically in relation to complicating disease states such as diabetes42, warrants further investigation.
Acknowledgments
Sources of Funding
This research was supported by the following grants: NSFC 30670879 (to L.T.), NIH 2R01HL-63828, American Diabetes Association Research Award 7-08-RA-98, American Heart Association Grant-in-Aid 0855554D (to X.L.M.) and the Emergency Medicine Foundation Career Development Grant (to W.B.L.).
Non-standard Abbreviations and Acronyms
- APN
Adiponectin
- DHE
dihydroethidium
- LAD
Left anterior descending coronary artery
- LV
Left ventricle
- LVEDP
Left ventricular end diastolic pressure
- MI
Myocardial infarction
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- RSG
Rosiglitazone
- SODM
Superoxide dismutase mimic
- WT
Wild type
Footnotes
Disclosures None
References
- 1.Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–1181. doi: 10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- 2.Forrat R, Sebbag L, Wiernsperger N, Guidollet J, Renaud S, De Lorgeril M. Acute myocardial infarction in dogs with experimental diabetes. Cardiovasc Res. 1993;27:1908–1912. doi: 10.1093/cvr/27.11.1908. [DOI] [PubMed] [Google Scholar]
- 3.Jagasia D, McNulty PH. Diabetes mellitus and heart failure. Congest Heart Fail. 2003;9:133–139. doi: 10.1111/j.1527-5299.2002.00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Ishibashi S, Perrey S, Osuga J, Gotoda T, Kitamine T, Tamura Y, Okazaki H, Yahagi N, Iizuka Y, Shionoiri F, Ohashi K, Harada K, Shimano H, Nagai R, Yamada N. Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol. 2001;21:372–377. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- 6.Jiang G, Dallas-Yang Q, Li Z, Szalkowski D, Liu F, Shen X, Wu M, Zhou G, Doebber T, Berger J, Moller DE, Zhang BB. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long-term treatment with PPARgamma agonists. Diabetes. 2002;51:2412–2419. doi: 10.2337/diabetes.51.8.2412. [DOI] [PubMed] [Google Scholar]
- 7.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, Chatterjee PK, Thiemermann C. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 9.Liu HR, Tao L, Gao E, Lopez BL, Christopher TA, Willette RN, Ohlstein EH, Yue TL, Ma XL. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res. 2004;62:135–144. doi: 10.1016/j.cardiores.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Wei J, Guan Y, Jin N, Mao J, Wang X. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces clinical inflammatory responses in type 2 diabetes with coronary artery disease after coronary angioplasty. Metabolism. 2005;54:590–597. doi: 10.1016/j.metabol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: main results from the Rosiglitazone Atherosclerosis Study. J Intern Med. 2007;261:293–305. doi: 10.1111/j.1365-2796.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 12.Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004;24:930–934. doi: 10.1161/01.ATV.0000124890.40436.77. [DOI] [PubMed] [Google Scholar]
- 13.Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Liapis CD, Alevizos M. Beneficial effects of rosiglitazone on novel cardiovascular risk factors in patients with Type 2 diabetes mellitus. Diabet Med. 2008;25:333–340. doi: 10.1111/j.1464-5491.2007.02375.x. [DOI] [PubMed] [Google Scholar]
- 14.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Loke YK, Furberg CD. Long-term Risk of Cardiovascular Events With Rosiglitazone: A Meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 16.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPAR{gamma} Ligands Increase Expression and Plasma Concentrations of Adiponectin, an Adipose-Derived Protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 17.Lipscombe LL. Thiazolidinediones: Do harms outweigh benefits? CMAJ. 2009;180:16–17. doi: 10.1503/cmaj.081713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 21.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 22.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 23.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 24.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice Lacking Adiponectin Show Decreased Hepatic Insulin Sensitivity and Reduced Responsiveness to Peroxisome Proliferator-activated Receptor {gamma} Agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 25.Hoo RL, Chow WS, Yau MH, Xu A, Tso AW, Tse HF, Fong CH, Tam S, Chan L, Lam KS. Adiponectin mediates the suppressive effect of rosiglitazone on plasminogen activator inhibitor-1 production. Arterioscler Thromb Vasc Biol. 2007;27:2777–2782. doi: 10.1161/ATVBAHA.107.152462. [DOI] [PubMed] [Google Scholar]
- 26.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta - oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 27.Spasojevic I, Chen Y, Noel TJ, Fan P, Zhang L, Rebouτas JS, Clair DK, Batinic-Haberle I. Pharmacokinetics of the potent redox-modulating manganese porphyrin, MnTE-2- PyP5+, in plasma and major organs of B6C3F1 mice. Free Radic Biol Med. 2008;45:943–949. doi: 10.1016/j.freeradbiomed.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: Role of S-nitrosation. PNAS. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 30.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe M, Takiguchi Y, Ichimaru S, Kaji S, Tsuchiya K, Wada K. Different effect of acute treatment with rosiglitazone on rat myocardial ischemia/reperfusion injury by administration method. Euro J Pharm. 2008;589:215–219. doi: 10.1016/j.ejphar.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 34.Huang PH, Sata M, Nishimatsu H, Sumi M, Hirata Y, Nagai R. Pioglitazone ameliorates endothelial dysfunction and restores ischemia-induced angiogenesis in diabetic mice. Biomed Pharmacother. 2008;62:46–52. doi: 10.1016/j.biopha.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Kojima S, Funahashi T, Sakamoto T, Miyamoto S, Soejima H, Hokamaki J, Kajiwara I, Sugiyama S, Yoshimura M, Fujimoto K, Miyao Y, Suefuji H, Kitagawa A, Ouchi N, Kihara S, Matsuzawa Y, Ogawa H. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667–668. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banga A, Unal R, Tripathi P, Pokrovskaya I, Owens RJ, Kern PA, Ranganathan G. Adiponectin translation is increased by the PPAR{gamma} agonists pioglitazone and {omega}-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296:E480–E489. doi: 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbate A, Biondi-Zoccai GG, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol. 2002;193:145–153. doi: 10.1002/jcp.10174. [DOI] [PubMed] [Google Scholar]
- 38.Von Bibra H, Diamant M, Scheffer PG, Siegmund T, Schumm-Draeger PM. Rosiglitazone, but not glimepiride, improves myocardial diastolic function in association with reduction in oxidative stress in type 2 diabetic patients without overt heart disease. Diab Vasc Dis Res. 2008;5:310–318. doi: 10.3132/dvdr.2008.045. [DOI] [PubMed] [Google Scholar]
- 39.Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 40.Tao L, Liu HR, Gao E, Teng ZP, Lopez BL, Christopher TA, Ma XL, Batinic-Haberle I, Willette RN, Ohlstein EH, Yue TL. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-{gamma} agonist in hypercholesterolemia. Circulation. 2003;108:2805–2811. doi: 10.1161/01.CIR.0000097003.49585.5E. [DOI] [PubMed] [Google Scholar]
- 41.Heusch G, Boengler K, Schulz R. Cardioprotection: Nitric Oxide, Protein Kinases, and Mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 42.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]