Abstract
Long-distance runners have higher high-density lipoprotein (HDL)-cholesterol concentrations and lower adiposity than sedentary men. Most cross-sectional studies claim that the runners’ elevated HDL-cholesterol is not due to the runners’ leanness. However, when cross-sectional studies use analysis of covariance (ANCOVA) to adjust for adiposity, or when they compare runners with lean sedentary men, they make an incorrect tacit assumption. They assume that the relationship between change in adiposity and change in HDL-cholesterol in men who have lost fat by running is the same as the cross-sectional difference in HDL-cholesterol between naturally fat and lean sedentary men. Regression slopes for HDL-cholesterol versus adiposity during and at the end of 1 year of running in 35 initially sedentary men suggest this assumption is incorrect; the increase in HDL-cholesterol that accompanies weight loss (−4.28 ± 1.0l mg/l00mL per kg/m2) is considerably greater than the increase in HDL-cholesterol that is associated with lower adiposity cross-sectionally (−0.78±0.46 mg/l00mL per kg/m2). These results suggest the following theory: long-distance runners have the HDL metabolism of men who are below their sedentary set-point weight rather than the HDL metabolism of men who are naturally lean without exercising or dieting. This theory was applied to data from 23 published comparisons between long-distance runners and sedentary men. The differences were highly correlated (r = 0.80) with the theory’s predictions (ie, the HDL-cholesterol differences predicted by applying the regression slope for change in HDL-cholesterol vs. change in adiposity to the average differences in adiposity between the runners and sedentary men). These analyses suggest that comparing long-distance runners to a reference population suitably matched for adiposity, and adjusting for adiposity by ANCOVA may each seriously underestimate the contribution of the runners’ leanness to their HDL concentrations. These results suggest that the elevated HDL-cholesterol concentrations of long-distance runners are primarily a phenomenon of reduced adiposity.
Long-distance runners have higher high-density lipoprotein (HDL)-cholesterol concentrations and lower adiposity than sedentary men [1–19]. Most cross-sectional studies claim that the runners’ elevated HDL-cholesterol is not due to the runners’ leanness. This claim is based on three observations: (1) long-distance runners have significantly higher HDL-cholesterol than sedentary men when adjusted for differences in adiposity by analysis of covariance (ANCOVA) [1,10,14]; (2) long-distance runners have significantly higher HDL-cholesterol than naturally lean sedentary men [18,20–22]; and (3) HDL-cholesterol and adiposity levels are only weakly correlated in cross-sectional samples of runners and sedentary men [6,17,23]. There are experimental data from longitudinal studies that contradict these cross-sectional observations. They suggest that metabolic processes associated with fat loss elevate HDL-cholesterol in runners. Specifically they show (1) a strong correlation between changes in HDL-cholesterol and adiposity in initially sedentary men who ran for 1 year [24,25]; (2) significantly increased HDL-cholesterol levels in sedentary men assigned at random to weight loss by diet or to weight loss by exercise in a l-year controlled tria1 [26]; and (3) significantly increased HDL-cholesterol during weight loss in many diet studies [27,28]. The conflicting interpretations of the cross-sectional and longitudinal data warrant further investigation.
STATISTICAL ANALYSES OF CROSS-SECTIONAL STUDIES
The discrepancy may occur because standard epidemiologic practices, including both statistical adjustment (ANCOVA) and the selection of matched controls, are inappropriate for cross-sectional comparisons between long-distance runners and sedentary men. Many runners are lean because they have lost fat. However, statistical adjustment by ANCOVA, and the selection of matched controls, are both based on the cross-sectional relationships between HDL-cholesterol and adiposity. Both assume that the relationship between change in adiposity and change in HDL-cholesterol in men who have lost fat by running is the same as the cross-sectional difference in HDL-cholesterol between naturally fat and lean sedentary men, and this is not true. The consequence (e.g., increased HDL) of physiologic change (weight loss) cannot be inferred from cross-sectional relationships. This can be shown using data from 35 initially sedentary men who were trained to run as part of a l-year study of exercise and lipoproteins [24,25] (includes only those men who had complete data on body composition, HDL-cholesterol and HDL2-mass at baseline and l-year who did not go on special diets to lose weight during the study, see Wood et al [25] for description of design and methods). The analyses appear in Table 1. The regression slopes for HDL-cholesterol versus adiposity at baseline (before regular exercise) describe the cross-sectional relationships between adiposity and HDL concentrations in sedentary men (designated β1S). The regression slopes for data collected at the end of the study, after 1 year of running, describe the cross-sectional relationships between adiposity and HDL concentrations in runners (designated β1R). The two cross-sectional regression slopes are compared with the regression slopes between change in HDL concentrations and change in adiposity (β2). This was done by subtracting β1S from β2, and β1R from β2, and dividing these differences by their corresponding standard errors to obtain t statistics.
Table 1.
Regression slopes (±SE) for HDL-cholesterol and HDL2-mass concentrations versus BMI, relative weight, and percent body fat in 35 initially sedentary men who participated in a l-year exercise program.
| BMI (kg/m2) | Relative weight (% of ideal) | Body fat (%) | |
|---|---|---|---|
| HDL-cholesterol | |||
| ΔHDL-cholesterol vs. ΔBMI (β2) | −4.28 ± 1.01§ | −92.36±21.99‡ | −1.14 ± 0.38† |
| HDL-cholesterol vs. BMI at baseline (β1S) | −0.78 ± 0.46 | −17.77±10.28 | −0.46 ± 0.27 |
| HDL-cholesterol vs. BMI after running year (β1R) | −0.57 ± 0.66 | −12.14±14.87 | −0.35 ± 0.36 |
| Test for significant difference (β2-β1S) | −3.50 ± 1.04† | −74.59±24.84† | −0.68 ± 0.46 |
| Test for significant difference (β2-β1R) | −3.71 ± 0.90‡ | −80.22±19.60‡ | −0.79 ± 0.33* |
| HDL2-mass | |||
| ΔHDL2-mass vs. ΔBMI (β2) | −16.87 ± 4.22‡ | −364.15±91.38‡ | −5.18 ± 1.49‡ |
| HDL2-mass vs. BMI at baseline (β1S) | −3.93 ± 1.76* | −93.50±38.92 | −1.81 ± 1.06 |
| HDL2-mass vs. BMI after running year (β1R) | −2.28 ± 2.26 | −56.35±50.96 | −1.78 ± 1.22 |
| Test for significant difference (β2-β1S) | −12.94 ± 4.92† | −207.05±107.18† | −3.37 ± 2.04 |
| Test for significant difference (β2-β1R) | −14.59 ± 3.80‡ | −307.80±82.48‡ | −3.40 ± 1.46* |
Significance levels are coded:
P<0.05;
P<0.01;
P<0.001;
P<0.0001.
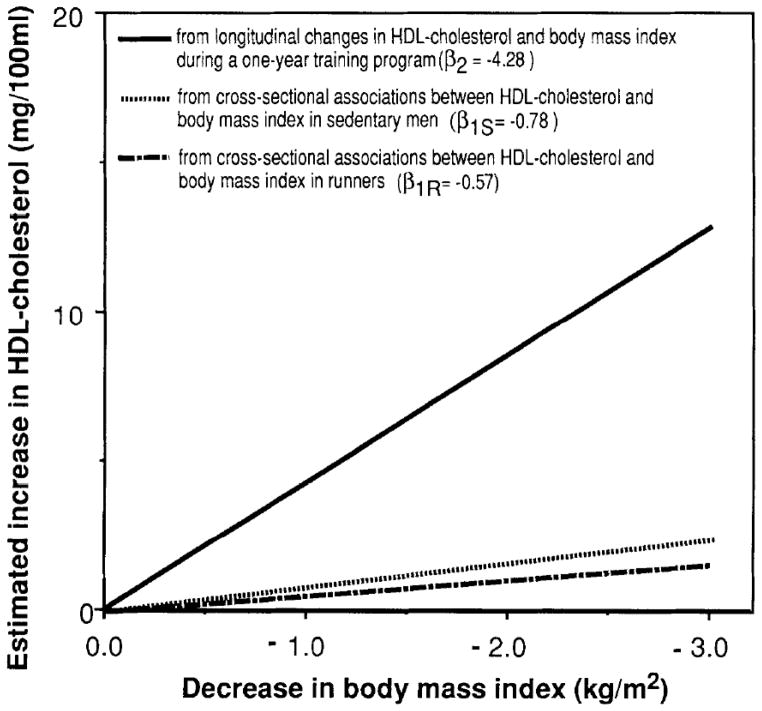
Table 1 shows that the differences (β2- β1S) and (β2- β1R) are negative, and that most are significant. The negative differences indicate that the cross-sectional relationships between HDL concentrations and adiposity in sedentary men (β1S) and in runners (β1R) underestimate the relationship between change in HDL concentrations and change in adiposity (β2). The increases in HDL-cholesterol and HDL2-mass that accompanies exercise-induced fat loss are considerably greater than the HDL-cholesterol and HDL2-mass differences between fat and lean sedentary men (β2>β1). The HDL-cholesterol difference is illustrated in Fig 1. Cross-sectionally, a 1-kg/m2 difference in body mass index (BMI) was associated with a HDL-cholesterol difference of only −0.78 mg/l00mL in sedentary men (β1S) and −0.57 mg/l00mL in the runners (β1R). These two estimates of β1 are both significantly less (P<0.0l) than the −4.28 mg/l00mL change per kg/m2 change observed longitudinally (β2).
Fig 1.
Three estimates of the expected change in HDL cholesterol with change in weight from data on 35 men who participated in a l-year running program. The effect of weight loss on HDL-cholesterol (β2) is underestimated by cross-sectional data (β1S and β1R).
EXERCISE AND WEIGHT SET-POINT EXERCISE AND WEIGHT SET-POINT
Multiple regression analysis was used to test whether HDL-cholesterol concentrations in runners are better predicted by current adiposity or rather by loss of adiposity since starting to run (Table 2). At the end of the l-year running program, plasma HDL-cholesterol concentrations were more strongly related to changes in BMI between baseline and 1 year than to body mass index at 1 year, and to changes in relative weight between baseline and 1 year than to relative weight at 1 year. That is, HDL-cholesterol levels depend more on the weight lost than the leanness achieved. These results, and the relationships between HDL concentrations and adiposity before, during, and at the end of 1 year of running (Table 1), suggest the following theory: long distance runners have the HDL metabolism of men who are below their sedentary weight (i.e., their theoretical sedentary set-point [29]) rather than the HDL metabolism of lean sedentary men who are at their usual weight. By sedentary weight set-point, we mean the postulated homeostatically regulated (usual) weight under sedentary conditions [29].
Table 2.
Multiple regression analyses (coefficients ± SE) showing that at the end of a l-year running program, the HDL-cholesterol concentrations of 35 men were more strongly related to the weight lost than to the leanness achieved. Dependent variable in both models is HDL-cholesterol (mg/dL) at the end of the l-year running program.
| Model 1 using BMI: | |
| Intercept | 72.85±15.34* |
| One-year change in BMI between baseline and the end of the study | −3.75±1.83* |
| BMI at the end of the study | −0.92±0.65 |
| Percent of the variance explained (R2) | 13.5% |
| Model 2 using relative weight: | |
| Intercept | 73.45±16.01* |
| One-year change in relative weight between baseline and the end of the study | −81.60±39.94* |
| Relative weight at the end of the study | −20.50±14.78 |
| Percent of the variance explained (R2) | 13.3% |
Significant at P < .05
REASSESSMENT OF CROSS-SECTIONAL STUDIES
The ANCOVA model assumes that the cross-sectional regression slope between HDL-cholesterol and adiposity (β1) can be used to adjust the runners’ and sedentary men’s HDL-cholesterol difference for their difference in adiposity. The product
| (Eq 1) |
estimates the amount of the HDL-cholesterol difference that is due to the difference in adiposity. This is subtracted from the runners’ and sedentary men’s average difference in HDL-cholesterol:
| (Eq 2) |
to estimate the amount of the lipoprotein difference that is not due to the adiposity difference (i.e., the adjusted difference in HDL-cholesterol).
We have shown in Table 1 that the relationship between change in adiposity and change in HDL-cholesterol in men who have lost fat by running (β2) is not the same as the cross-sectional difference in HDL-cholesterol between naturally fat and lean sedentary men (β1). Since β2 > β1, the ANCOVA model is expected to underestimate substantially the HDL-cholesterol difference that is due to the leanness of the runners. The weight set-point model suggests that the regression slope between change in HDL-cholesterol and change adiposity should be used to adjust the runners’ and sedentary men’s HDL-cholesterol difference for their difference in adiposity. However, this requires knowledge of the adiposity of the individual runners under sedentary conditions. Although this is not known for individual runners, the average BMI of the sedentary men may provide a reasonable estimate of the average BMI of the runners under sedentary conditions, provided that the sedentary men were selected to represent the relevant pool of men prior to running long distances. Under the weight set-point theory, the part of the HDL-cholesterol difference that is attributable to the long-distance runners’ reduced adiposity is;
| (Eq 3) |
where β2 is the regression slope between change in HDL-cholesterol and change in adiposity as determined from longitudinal data. The remainder:
| (Eq 4) |
is the adjusted difference in HDL-cholesterol concentrations between long-distance runners and sedentary men (i.e., with the effects of their difference in adiposity removed).
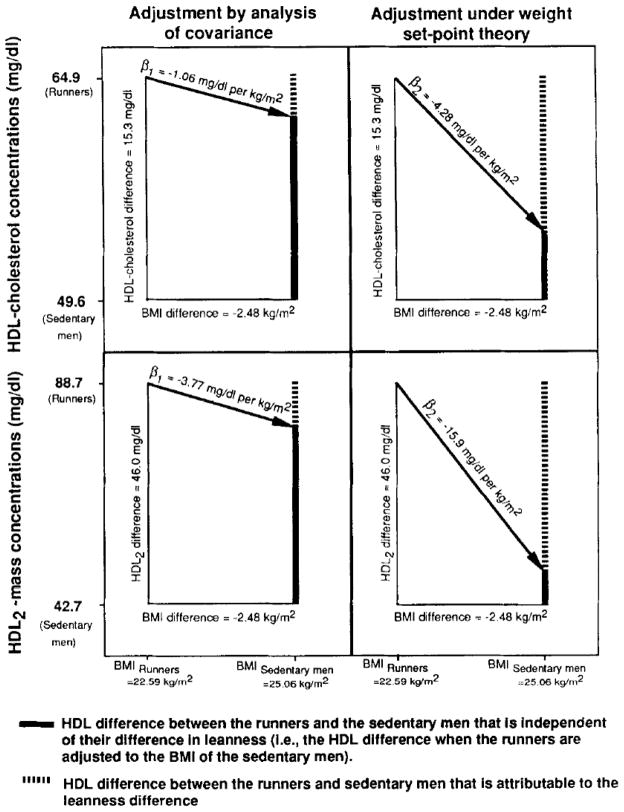
For example, Williams et al [10] reported that mean HDL-cholesterol concentrations were 15.33 mg/l00mL higher in runners than sedentary men and that the runners were also −2.48 kg/m2 leaner. They also observed that a 1-kg/m2 decrease in BMI was associated with a 1.065 mg/100 mL increase in HDL-cholesterol (i.e., β1 = −1.065 mg/l00mL per kg/m2) in these cross-sectional data. Under the ANCOVA model (Eq 1 and 2), they estimated that 2.64 mg/100mL of the HDL-cholesterol difference was due to the adiposity difference, and that the adjusted difference in HDL-cholesterol between the runners and the sedentary men was:
The weight set-point adjustment assumes that the runners are 2.48 kg/m2 below their sedentary adiposity level, and that β2 = −4.28 mg/l00mL per kg/m2 (see Table 1). Therefore, under the weight set-point model (Eq 3 and 4), we estimate that 10.61 mg/l00mL of the HDL-cholesterol difference was due to the reduced adiposity of the runners, and that the adjusted difference in HDL-cholesterol between the runners and the sedentary men is
Therefore, as shown in Fig 2, differences in mean adiposity explain only 17% of the runners’ and sedentary men’s difference in HDL-cholesterol under the ANCOVA model, but 70% of the HDL-cholesterol difference under the weight set-point model. Figure 2 also shows that the ANCOVA model attributes much less of HDL2 difference to the runners’ and sedentary men’s difference in adiposity (20% of the HDL2 difference) than does the weight set-point model (86%).
Fig 2.
Statistical adjustment of the mean difference in HDL-cholesterol and HDL2 concentrations between 12 long-distance runners and 64 sedentary [10] men under the ANCOVA and weight set-point models. The drop in the regression line that occurs between the runners’ and sedentary men’s mean BMI designates the portion of the HDL2 difference that is attributable to leanness. The weight set-point model replaces the cross-sectional regression slope used in the ANCOVA calculation with the regression slope for change in HDL versus change in BMI. Note that a substantially greater proportion of the HDL difference is attributed the runner’s leanness under the weight set-point model.
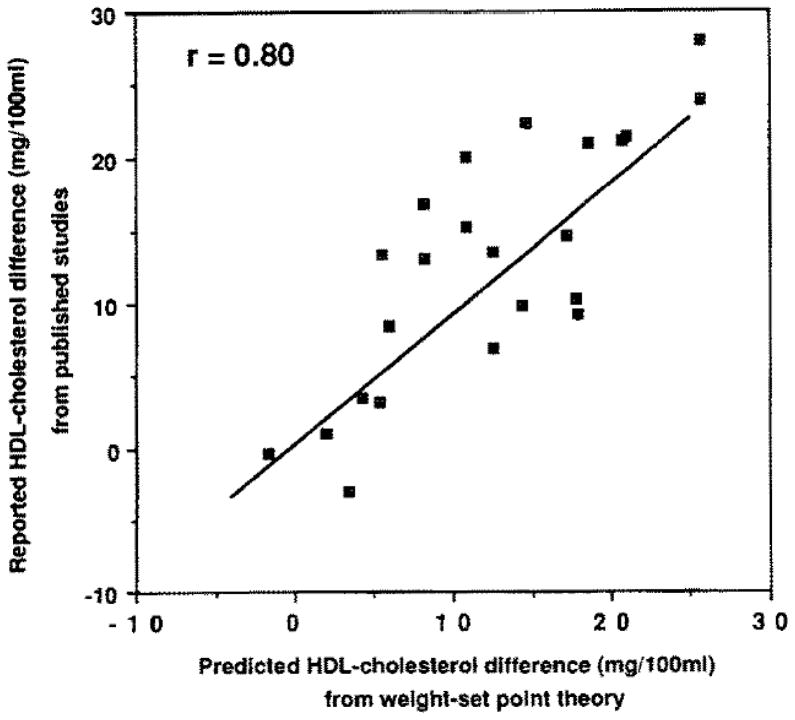
The weight set-point theory of long-distance runners can be tested using data previously published by others. There are 23 published comparisons between long-distance runners and sedentary men (Table 3). If the theory is valid, then most of the HDL difference should be predicted by applying the regression slope for change in HDL versus change in adiposity to the runners and nonrunners difference in adiposity (Eq 3). From the 35 men who participated in our l-year training study, our estimates of the β2 coefficients for HDL-cholesterol are −1.34 mg/100mL per kg change in weight, −1.14 mg/100mL change per 1% change in body fat, −4.28 mg/l00mL change per kg/m2 change in BMI, −92.36 mg/l00mL change per 1-U change in relative weight, and −103.48 mg/l00mL change per 1-U change in Broca’s index {weight (kg)/[height (cm) − l00]}. The HDL difference predicted by the model correlates strongly (r = .80) with the published differences (Table 3). The regression line between the observed and predicted differences has a slope close to one and an intercept close to zero (Fig 3). In contrast, the average distance run does not correlate with the HDL-cholesterol differences between runners and sedentary men {r = .08 when the HDL-cholesterol differences and distances run reported by Martin et al (7.0 mg/l00mL v 137 km/wk) [23], Nakamura et al (13.6 mg/l00mL vs. 20 km/wk) [21],” and Nikkila et al (19.1 mg/l00mL vs. 115 km/wk) [22], are included with 16 of the studies listed in Table 3).
Table 3.
Cross-sectional studies of HDL-cholesterol concentrations in long-distance runners and sedentary men.
| Difference between exercisers and sedentary men | ||||||
|---|---|---|---|---|---|---|
| ΔAdiposity | ΔHDL-cholesterol (mg/100ml) | |||||
| Sample Size: Exercise/Control | Training Level (km/wk) | Measurement | Difference Δ ± SE | Predicted difference from the Regression Slopes of Table 1 and ΔAdiposity | Observed Difference. Δ±SE | |
| Hartung [1] | 15/13 | average 32 | weight (kg) | 1.20 ± 3.59 | −1.6 | −0.4 ± 5.5 |
| Rifai [2] | 9/7 | at least 48 | weight (kg) | −1.49 ± 6.61 | 2.0 | 1.0 ± 5.1 |
| Thompson [3] | 8/9 | 3 times/wk | weight (kg) | −2.49 ± 4.46 | 3.3 | 3.0 ± 5.1 |
| Marniemi [4] | 26/35 | 10 h/wk | weight (kg) | −3.10 ± 2.19 | 4.2 | 3.5 ± 2.2 |
| Clarkson [5] | 6/17 | average 113 | weight (kg) | −3.92 ± 3.61 | 5.3 | 3.2 ± 5.0 |
| Adner [6] | 50/43 | average 56 | rel. weight | −0.06 ± 0.02 | 5.5 | 13.4 ± 2.5 |
| Hartung [1] | 16/13 | average 69 | weight (kg) | −4.40 ± 3.79 | 5.9 | 8.5 ± 5.3 |
| Berg [7] | 18/18 | 10.8 hr/wk | Broca’s index | −0.08 ± 0.03 | 8.1 | 16.9 ± 3.1 |
| Hamalainen [8] | 20/20 | 19 to 104 | BMI | −1.90 ± 1.03 | 8.1 | 13.1 ± 3.7 |
| Thompson [9] | 20/14 | average 113 | weight (kg) | −8.00 ± 2.37 | 10.7 | 20.0 ± 3.8 |
| Williams [10] | 12/64 | average 64 | BMI | −2.50 ± 0.70 | 10.7 | 15.3 ± 3.8 |
| Schnabel [11] | 19/20 | mid-distance | Broca’s index | −0.12 ± 0.03 | 12.4 | 6.9 ± 3.3 |
| Lehtonen [12] | 23/15 | at least 25 | Broca’s index | −0.12 ± 0.03 | 12.4 | 13.6 ± 4.4 |
| Squires [13] | 8/15 | Average 18 | weight (kg) | −10.60 ± 4.81 | 14.2 | 9.8 |
| Schnabel [10] | 1 l/20 | long-distance | Broca’s index | −0.14 ± 0.03 | 14.5 | 22.4 ± 4.4 |
| Hartung [14] | 85/74 | average 18 | weight (kg) | −12.70 ± 1.70 | 17.0 | 14.7 ± 2.5 |
| Tsopanakis [15] | 13/24 | long-distance | Broca’s index | −0.17 ± 0.02 | 17.6 | 10.3 ± 2.8 |
| Schriewer [16] | 45/45 | average 70 | Broca’s index | −0.18 | 18.3 | 9.2 ± 3.8 |
| Wood [17] | 41/147 | average 63 | rel. weight | −0.20 ± 0.01 | 18.5 | 21.0 ± 2.2 |
| Squires [13] | 8/15 | average 60 | weight (kg) | −15.30 ± 5.66 | 20.5 | 21.1 |
| Hartung [14] | 59/74 | average 64 | weight (kg) | −15.55 ± 1.81 | 20.9 | 21.5 ± 2.5 |
| Seals [18] | 14/12 | average 89 | weight (kg) | −19.0 ± 2.83 | 25.5 | 24.0 ± 5.0 |
| Thompson [19] | 9/l0 | 68 to 113 | weight (kg) | −19.0 ± 6.60 | 25.5 | 28.0 ± 5.6 |
Fig 3.
Reported average differences in HDL-cholesterol between long-distance runners and sedentary men versus the predicted differences in HDL-cholesterol concentrations under the weight set-point model. Twenty-three cross-sectional comparisons are displayed.
WEIGHT SET-POINT THEORY AND HDL-METABOLISM IN RUNNERS
Lipoprotein lipase hydrolyzes triglycerides of chylomicron and very-low-density lipoprotein (VLDL) particles [30]. During this process (lipolysis), the free cholesterol, apolipoproteins, and phospholipids that reside on the surfaces of these particles are taken up by circulating HDL [30]. Runners have high lipoprotein lipase activity and therefore catabolize chylomicron and VLDL particles more rapidly than sedentary men [31]. There are at least two explanations of how more rapid catabolism of these particles could increase plasma HDL-cholesterol concentrations in long-distance runners: (1) free cholesterol and other surface components may be transferred to HDL at a higher rate when chylomicron and VLDL particles are catabolized more rapidly [22,31]; (2) HDL-cholesteryl ester may be transferred to VLDL and chylomicron particles at a slower rate because the size of the pool of VLDL and chylomicron particles is reduced, causing HDL-cholesterol to accumulate [32]. Running also decreases the measured activity of hepatic lipase [10,33], an enzyme that hydrolyzes HDL-phospholipids [34]. This may also cause HDL-cholesterol to accumulate in plasma, because an increase in the ratio of HDL-phospholipid to HDL-cholesterol may inhibit the transfer of HDL-cholesterol to hepatocytes [35,36].
Previous discussions have emphasized the adaptations of skeletal muscles to prolonged physical activity, particularly the higher lipoprotein lipase activity of muscles in long-distance runners [31,37–40]. Our results suggest that the elevated HDL-cholesterol concentrations of long-distance runners appear to be primarily a phenomenon of reduced adiposity rather than altered musculature. We propose that it is the higher adipose tissue lipoprotein lipase activity that is primarily responsible for the greater catabolic rate of chylomicrons and VLDL particles and their lower plasma concentrations. This possibility has been ignored in earlier exercise studies, even when clearly indicated by the data presented. For example, the data presented by Nikkila et al show that the increase in lipoprotein lipase activity of adipose tissue is greater, and contributes more substantially to overall increases in lipoprotein lipase activity of long-distance runners than does the higher lipase activity of the skeletal muscle [22]. Per gram of tissue, adipose tissue lipoprotein lipase activity was 175% higher in the long-distance runners than in the sedentary men they studied; this difference is much larger than the difference they observed between the runners’ and the sedentary men’s skeletal muscle lipoprotein lipase activities (67% higher in long-distance runners). Using rough approximations of muscle and fat body mass from published references, Nikkila et al estimated that the total lipoprotein lipase activity of all adipose tissue and skeletal muscle was 125.4 mmol free fatty acids (FFA) • h−1 in long-distance runners and 59.6 mmol FFA • h−1 in sedentary men. Adipose tissue lipoprotein lipase activity accounted for approximately 79% of this difference [22]. Nikkila et al also reported that distance run and plasma HDL-cholesterol concentrations correlated significantly with adipose tissue lipoprotein lipase activity but not skeletal muscle lipoprotein lipase activity in their study [22]. The greater increase in the lipoprotein activity of the adipose tissue vis-a-vis the muscle tissue was never made explicit by the authors, nor its importance discussed [22,31].
The observation that the increase in adipose tissue lipoprotein lipase activity is greater than the increase in skeletal muscle lipoprotein lipase activity is consistent with runners’ greater utilization of energy substrates from adipose tissue than muscle tissue during running. Oxidation of FFA released by the adipocytes is estimated to contribute 25% to 90% of the energy spent during prolonged endurance exercise [41]. In contrast, uptake of VLDL-triglycerides and use of intramuscular triglyceride stores are estimated to provide less than 15% of the energy used by the muscle during prolonged exercise [42,43] Thus, the energy required to run long distances is likely to promote a greater increase in the hydrolysis of triglyceride-rich lipoproteins by adipose tissue (replacing fatty acids released by adipocytes during exercise) than by muscle tissue.
Why should the HDL-cholesterol concentrations of long distance runners be determined by their reduced adiposity relative to their weight set-point rather than their current adiposity? The increase in the runner’s adipose tissue lipoprotein lipase activity may be proportional to the level of depletion of their adipocyte triglyceride stores that has occurred since the men started running. If true, then running should affect the relationship of lipoprotein lipase activity with percent body fat and fat cell diameter. In sedentary populations, lipoprotein lipase activity correlates positively with percent body fat and fat cell diameter. These relationships would be weakened if long-distance runners who lose the most weight also increase adipose tissue lipoprotein lipase activity and decrease adipocyte diameter. Sevard et al [44] has in fact found that the relationships of adipose tissue lipoprotein lipase activity to fat cell diameter and percent body fat in sedentary men (r = 0.79 and r = 0.47, respectively) differ from those of long-distance runners (r = −0.03 and r = −0.38) studied cross-sectionally.
CAVEATS AND LIMITATIONS
Our proposed theory attributes 64% of the variance in the mean HDL differences between runners and sedentary men to the leanness of runners. The analysis invoked several assumptions: (1) the average adiposity and HDL-cholesterol levels of sedentary men provides a reasonable estimate of the runners’ values under sedentary conditions; (2) the relationship between l-year changes in HDL-cholesterol and adiposity can be extrapolated to the longer running careers of distance runners. Random samples of sedentary men may not precisely represent the relevant pool of men before running long distances because men who take up running may have initially less fat and higher HDL-cholesterol levels [45,46]. The second assumption remains to be verified. The unexplained variation in HDL levels in the 23 studies (36% of the variance) may be due to inaccuracies in the model’s parameter estimates and random variation.
Other physiological effects of exercise, including training induced changes in skeletal muscle of runners and their high calorie flux may also contribute to the remaining variation. Keins and Lithell compared lipoprotein levels of arterial and venous blood samples taken in trained and untrained leg muscles [47]. They found that the mean arteriovenous HDL-cholesterol differences were significantly greater in the trained muscles than the untrained muscles. In another study, Sopko et al reported that a combination of increased calorie intake and exercise increased plasma HDL-cholesterol without weight loss [48]. The effects of hypercaloric status on HDL-cholesterol are poorly understood. Calculations by Nikkila suggest that chylomicrons provide 5 to 10 times more surface phospholipids and cholesterol for HDL than does endogenous VLDL [30]. Therefore, the potential for higher muscle and adipose tissue lipoprotein lipase activities to increase HDL-cholesterol concentrations may be greatest when chylomicron turnover is also increased. Although our theory does not preclude contribution of these other factors, training-induced changes in skeletal muscles and high calorie flux do not explain why previously overweight marathon runners have higher HDL-cholesterol levels than marathon runners who were never overweight [49].
CONCLUSIONS
We have presented evidence to suggest that the standard epidemiologic practices of comparing long-distance runners with a reference population suitably matched for adiposity and adjusting for adiposity by analysis of covariance may seriously underestimate the importance of the runners’ leanness on their HDL concentrations. These practices incorrectly assume that the relationship between change in adiposity and change in HDL-cholesterol in men who have lost fat by running is the same as the cross-sectional difference in HDL-cholesterol between naturally fat and lean sedentary men. Comparisons between long-distance runners and lean sedentary men in the studies by Letac et al [20], Nakamura et al [21], Nikkila et al [22], and Seals et al [18] may involve individuals of comparable leanness, but this approach negates differences in their adipose tissue morphology and metabolism, e.g., long-distance runners tend to have smaller fat cells [22,44,50] higher adipose tissue lipoprotein lipase activity [22,44] and higher basal and insulin-stimulated rates of glucose conversion to triglycerides [44]. The observations by Savard et al [44] of a different relationship between adiposity and adipose tissue lipase activity in sedentary men and long-distance runners is consistent with our criticism of the ANCOVA adjustment in cross-sectional studies of runners and sedentary men. Their observation may also explain why several cross-sectional studies have found that the correlation between HDL-cholesterol and adiposity may be different in sedentary men and long-distance runners [17].
Men who are below their usual weight by dieting generally sustain their fat loss only by continuing to restrict their calorie intake. Schwartz and Brunzell have hypothesized that increased lipoprotein lipase activity may be an important regulatory response following loss of adiposity below usual weight [51]. The increase in adipose tissue lipoprotein lipase activity in these men may primarily serve to return adipose mass and fat cell size to that specified by the postulated set-point for body weight [29,51]. However, men who have lost weight by long-distance running are able to sustain the reductions in fat cell size on unrestricted diets that contain 40% to 60% more calories than sedentary men who are at stable weight [52]. Long-distance runners and men who have lost weight by dieting may share the same unstable metabolic state below their theoretical set-point weight, but whereas dieters often return to their initial weight, long-distance runners are able to sustain their reduced weight and maintain increased HDL-cholesterol concentration in conjunction with a high caloric intake.
Acknowledgments
The author wishes to thank Drs Peter D.S. Wood and William L. Haskell for providing the data used in these analyses.
Supported by National Institutes of Health Grant No. HL-02183 from the National Heart, Lung and Blood Institute of the National Institutes of Health, and was conducted at the Lawrence Berkeley Laboratory (Department of Energy Contract No. DE-AC03-76SFOOO98 to the University of California).
References
- 1.Hartung GH, Foreyt JP, Mitchell RE, et al. Effect of alcohol intake on high density lipoprotein cholesterol levels in runners and inactive men. JAMA. 1983;249:747–750. [PubMed] [Google Scholar]
- 2.Rifai N, King ME, De Meersman R, et al. Apolipoprotein and lipoprotein cholesterol concentrations in trained and sedentary males. Clin Chim Acta. 1987;163:113–l17. doi: 10.1016/0009-8981(87)90040-4. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CE, Thomas TR, Araujo J, et al. Response of HDL-cholesterol, apolipoprotein A-I, and LCAT to exercise withdrawal. Atherosclerosis. 1985;54:65–73. doi: 10.1016/0021-9150(85)90154-6. [DOI] [PubMed] [Google Scholar]
- 4.Marniemi J, Dahlstrom S, Kvist M, et al. Dependence of serum lipids and lecithin:cholesterol acyltransferase levels on physical training of young men. Eur J Appl Physiol. 1982;49:25–35. doi: 10.1007/BF00428960. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson PM, Hintermister R, Filloyow M, et al. High density lipoprotein cholesterol in young adult weight lifters, runners, and untrained subjects. Hum Biol. 1981;53:251–257. [PubMed] [Google Scholar]
- 6.Adner MM, Castelli WP. Elevated high density lipoprotein levels in marathon levels. JAMA. 1980;243:534–536. [PubMed] [Google Scholar]
- 7.Berg A, Frey I, Keul J. Apolipoprotein profile in healthy males and its relation to maximum aerobic capacity. Clin Chim Acta. 1986;161:165–171. doi: 10.1016/0009-8981(86)90210-x. [DOI] [PubMed] [Google Scholar]
- 8.Hamalainen E, Tikkanen H, Harkonen M, et al. Serum lipoproteins, sex hormones and sex hormone binding globulin in middle-aged men of different physical fitness and risk of coronary heart disease. Atherosclerosis. 1987;67:155–162. doi: 10.1016/0021-9150(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PD, Lazarus B, Cullinane E, et al. Exercise, diet or physical characteristics as determinants of HDL-levels in endurance athletes. Atherosclerosis. 1983;46:333–339. doi: 10.1016/0021-9150(83)90182-x. [DOI] [PubMed] [Google Scholar]
- 10.Williams PT, Krauss RM, Wood PD, et al. Lipoprotein subfractions of runners and sedentary men. Metabolism. 1986;35:45–52. doi: 10.1016/0026-0495(86)90094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnabel A, Kindermann W. Effects of maximal oxygen uptake and different forms of physical training on serum lipoproteins. Eur J Appl Physiol. 1982;48:263–277. doi: 10.1007/BF00422987. [DOI] [PubMed] [Google Scholar]
- 12.Lehtonen A, Viikari J. Serum triglyceride and cholesterol and high-density lipoprotein cholesterol in highly physically active men. Acta Med Stand. 1978;204:111–114. doi: 10.1111/j.0954-6820.1978.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 13.Squires RW, Bove AA, Kottke BA, et al. Exercise training and the apolipoprotein A-I to HDL-cholesterol. J Cardiopulm Rehab. 1987;7:481–486. [Google Scholar]
- 14.Hartung GH, Foreyt JP, Mitchell RE, et al. Relation of diet to high density lipoprotein cholesterol in middle aged marathon runners, joggers, and inactive men. N Engl J Med. 1980;302:357–361. doi: 10.1056/NEJM198002143020701. [DOI] [PubMed] [Google Scholar]
- 15.Tsopanakis C, Kotsarellis D, Tsopanakis AD. Lipoprotein and lipid profiles of elite athletes in Olympic sports. Int J Sports Med. 1986;7:316–321. doi: 10.1055/s-2008-1025783. [DOI] [PubMed] [Google Scholar]
- 16.Schriewer H, Gunnewig V, Jung K, et al. The influence of a 100 km run on the composition of HDL. J Clin Chem Biochem. 1982;20:533–536. doi: 10.1515/cclm.1982.20.8.533. [DOI] [PubMed] [Google Scholar]
- 17.Wood PD, Haskell WL, Klein H, et al. The distribution of plasma lipoproteins in middle-aged male runners. Metabolism. 1976;25:1249–1257. doi: 10.1016/s0026-0495(76)80008-x. [DOI] [PubMed] [Google Scholar]
- 18.Seals DR, Allen WK, Hurley BF, et al. Elevated high density lipoprotein cholesterol levels in older endurance athletes. Am J Cardiol. 1984;54:390–393. doi: 10.1016/0002-9149(84)90203-0. [DOI] [PubMed] [Google Scholar]
- 19.Thompson PD, Kantor MA, Cullinane EM, et al. Postheparin plasma lipolytic activities in physically active and sedentary men after varying and repeated doses of intravenous heparin. Metabolism. 1986;35:999–1004. doi: 10.1016/0026-0495(86)90035-1. [DOI] [PubMed] [Google Scholar]
- 20.Letac B, Decorbiere P, Barthes P, et al. Effects of intensive physical training on blood lipids: A study in long-distance runners. Nouv Presse Med. 1981;10:403–406. [PubMed] [Google Scholar]
- 21.Nakamura N, Uzawa H, Maeda H, et al. Physical fitness: Its contribution to serum high density lipoproteins. Atherosclerosis. 1983;48:173–183. doi: 10.1016/0021-9150(83)90104-1. [DOI] [PubMed] [Google Scholar]
- 22.Nikkila EA, Taskinen MR, Rehunen S, et al. Lipoprotein lipase activity in adipose tissue and skeletal muscle of runners: relation to serum lipoproteins. Metabolism. 1978;27:1661–1671. doi: 10.1016/0026-0495(78)90288-3. [DOI] [PubMed] [Google Scholar]
- 23.Martin RP, Haskell WL, Wood PD. Blood chemistry and lipid profiles of elite distance runners. Ann NY Acad Sci. 1977;301:346–370. doi: 10.1111/j.1749-6632.1977.tb38212.x. [DOI] [PubMed] [Google Scholar]
- 24.Williams PT, Wood PD, Krauss RM, et al. Does weight loss cause the exercise-induced increase in plasma high density lipoproteins? Atherosclerosis. 1983;47:173–185. doi: 10.1016/0021-9150(83)90153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood PD, Haskell WL, Blair SN, et al. Increased exercise level and plasma lipoprotein concentrations: A one-year, randomized, controlled study in sedentary, middle aged men. Metabolism. 1983;32:31–39. doi: 10.1016/0026-0495(83)90152-x. [DOI] [PubMed] [Google Scholar]
- 26.Wood PD, Stefanick ML, Dreon D, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 27.Sorbris R, Petersson BG, Nilsson-Ehle P, et al. Effect of weight reduction on plasma lipoproteins and adipose tissue metabolism in obese subjects. Eur J Clin Invest. 1981;11:491–8. doi: 10.1111/j.1365-2362.1981.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 28.Streja DA, Marliss EB, Steiner G. The effects of prolonged fasting on plasma triglyceride kinetics in man. Metabolism. 1977;26:505–516. doi: 10.1016/0026-0495(77)90094-4. [DOI] [PubMed] [Google Scholar]
- 29.Keesey RE. The physiological regulation of body weight and the issue of obesity. Med Clin North Am. 1989;73:15–27. doi: 10.1016/s0025-7125(16)30689-7. [DOI] [PubMed] [Google Scholar]
- 30.Nikkill EA. HDL in relation to the metabolism of triglyceride rich lipoproteins. In: Miller NE, Miller GJ, editors. Clinical and Metabolic Aspects of High-Density Lipoproteins. Amsterdam, The Netherlands: Elsevier Science; 1984. pp. 217–245. [Google Scholar]
- 31.Nikkila EA. Role of lipoprotein lipase in metabolic adaptation to exercise training. In: Borensztajn J, editor. Lipoprotein Lipase. Chicago, IL: Evener; 1987. pp. 187–199. [Google Scholar]
- 32.Williams PT, Krauss RM, Vranizan KM, et al. The effects of exercise-induced weight loss on plasma low-density-lipoprotein subtraction concentrations in men. Arteriosclerosis. 1989;9:623–632. doi: 10.1161/01.atv.9.5.623. [DOI] [PubMed] [Google Scholar]
- 33.Stefanick ML, Terry RB, Haskell WL, et al. Relationships of changes in post-heparin hepatic and lipoprotein lipase activity to HDL-cholesterol changes following weight loss achieved by dieting versus exercise. In: Gallo L, editor. Cardiovascular Disease: Molecular and Cellular Mechanisms, Prevention, and Treatment. New York, NY: Plenum; 1987. pp. 61–68. [Google Scholar]
- 34.Grosser J, Schrecker O, Greten H. Function of hepatic triglyceride lipase in lipoprotein metabolism. J Lipid Res. 1981;22:437–442. [PubMed] [Google Scholar]
- 35.Miller NE. Current concepts of the role of HDL in reverse cholesterol transport. In: Miller NE, Miller GJ, editors. Clinical and Metabolic Aspects of High-Density Lipoproteins. Amsterdam, The Netherlands: Elsevier Science; 1984. pp. 187–216. [Google Scholar]
- 36.Rothblat GH, Arbogast LY, Ray EK. Stimulation of esterified cholesterol accumulation in tissue culture cells exposed to high density lipoproteins enriched in free cholesterol. J Lipid Res. 1978;19:350–358. [PubMed] [Google Scholar]
- 37.Kiens B, Lithell H. Lipoprotein metabolism related to adaptations in human skeletal muscle. Clin Physiol. 1985;5:108. [Google Scholar]
- 38.Mole PA, Oscai LB, Holloszy JO. increase in levels of palmitzl CoA synthetase, carnitine palmityltransferase, and palmityl CoA dehydrogenase and in the capacity to oxidize fatty acids. J Clin Invest. 1971;50:2323–2330. doi: 10.1172/JCI106730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svedenhag J, Lithell H, Juhlin-Dannfelt A, et al. Increase in skeletal muscle lipoprotein lipase following endurance training in man. Atherosclerosis. 1983;49:203–207. doi: 10.1016/0021-9150(83)90198-3. [DOI] [PubMed] [Google Scholar]
- 40.Taskinen M-J, Nikkila EA, Rehunen S, et al. Effects of acute vigorous exercise on lipoprotein lipase activity of adipose tissue and skeletal muscle of physically active men. Artery. 1980;6:471–483. [PubMed] [Google Scholar]
- 41.Gollnick PD. Free fatty acid turnover and the availability of substrates as a limiting factor in prolonged exercise. Ann NY Acad Sci. 1977;301:64–71. [Google Scholar]
- 42.Have1 RJ, Pernow B, Jones NL. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol. 1967;23:90–96. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- 43.Issekutz B, Miller HI, Paul P, et al. Source of fat oxidation in exercising legs. Am J Physiol. 1964;207:583–589. doi: 10.1152/ajplegacy.1964.207.3.583. [DOI] [PubMed] [Google Scholar]
- 44.Savard R, Despres JP, Deshaies Y, et al. Adipose tissue lipid accumulation pathways in marathon runners. Int J Sports Med. 1985;6:287–291. doi: 10.1055/s-2008-1025853. [DOI] [PubMed] [Google Scholar]
- 45.Wood PD, Haskell WL, Stern MP, et al. Plasma lipoprotein distributions in male and female runners. Ann NY Acad Sci. 1977;301:748–763. doi: 10.1111/j.1749-6632.1977.tb38244.x. [DOI] [PubMed] [Google Scholar]
- 46.Williams PT, Wood PD, Haskell WL, et al. The effects of running mileage and duration on plasma lipoprotein concentrations. JAMA. 1982;247:2674–2679. [PubMed] [Google Scholar]
- 47.Keins B, Lithell H. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J Clin Invest. 1989;83:558–564. doi: 10.1172/JCI113918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sopko G, Leon AS, Jacobs DR, et al. The effects of exercise and weight loss on plasma lipids in young obese men. Metabolism. 1985;34:227–236. doi: 10.1016/0026-0495(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 49.Williams PT. Weight set point theory predicts HDL-cholesterol levels in previously-obese marathon runners. Int J Obesity. 1990;14:421–427. [PubMed] [Google Scholar]
- 50.Desprts JP, Savard R, Trembloy A, et al. Adipocyte diameter and lipolytic activity in marathon runners: Relationship with body fatness. Eur J Appl Physiol. 1983;51:223–230. [Google Scholar]
- 51.Schwartz RS, Brunzell JD. Increase of adipose tissue lipoprotein lipase activity with weight loss. J Clin Invest. 1981;67:1425–1430. doi: 10.1172/JCI110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blair SN, Ellsworth NM, Haskell WL, et al. Comparison of the nutrient intake in middle-aged men and women runners and controls. Med Sci Sports Exer. 1981;13:310–315. [PubMed] [Google Scholar]