Abstract
Native oxide surfaces of stainless steel 316L and Nitinol alloys and their constituent metal oxides namely, nickel, chromium, molybdenum, manganese, iron and titanium were modified with long chain organic acids to better understand organic film formation. The adhesion and stability of films of octadecylphosphonic acid, octadecylhydroxamic acid, octadecylcarboxylic acid and octadecylsulfonic acid on these substrates was examined in this study. The films formed on these surfaces were analyzed by diffuse reflectance infrared Fourier transform spectroscopy, contact angle goniometry, atomic force microscopy and matrix assisted laser desorption ionization mass spectrometry. The effect of the acidity of the organic moiety and substrate composition on the film characteristics and stability is discussed. Interestingly, on the alloy surfaces, the presence of less reactive metal sites does not inhibit film formation.
Introduction
Self assembled monolayers (SAMs) of thiols on gold and silanes on silicon have been model systems for numerous applications including adhesion1, corrosion inhibition2, biomaterials3, 4, biosensors5, wettability6, click-chemistry7 and organic electronics.8 The structure and conformation of thin films of thiols on gold are based on the strong interaction between sulfur and gold. These SAMs present functional tail groups in a consistent manner allowing the films to be used in a variety of applications9–12. While forming SAMs on metals and alloys relevant to a particular biomedical or industrial application represents a more straightforward approach to functionalizing these materials, the chemistry of these surfaces has not been as well-studied, in most cases, and is therefore not predictable.
Modification of metal oxide surfaces by organic monolayer formation has been studied since Nuzzo and Allara’s seminal work in the 1980’s.13 Since then, many metal oxides such as aluminium13, 14, copper14–16, nickel8, 17, titanium16, 18, 19, zirconium, 18, 20, tantalum21, silicon,22, 23 mica24, silver14, 16, iron2, 16, 25, manganese and chromium26 have been used as substrates to form SAMs. The choice of head group is critical for the stability of SAMs. Long chain aliphatic molecules with head groups such as silanes,27, 28 thiols,29, 30 carboxylic acid,13, 31–35, phosphonic acid,36–39, sulfonic acid,39, 40 and hydroxamic acid16, 41 have been utilized on these metal and metal oxide substrates. Despite a significant number of reports of SAMs on metal oxides, it is difficult to predict which head group or acid will result in a complete monolayer on a specific metal substrate. This has been proven to be even more difficult on alloy oxides, where little work has been done.27, 36, 37, 42–44
Stainless steel 316L is a low-carbon austenitic steel which is used as surgical implant material. Like all stainless steels, it contains a minimum of 12% Cr to aid in the formation of protective surface layer which resists corrosion in a wide range of environments, but is also less susceptible to sensitization and more resistant to chloride-assisted corrosion due to its lower carbon content and alloying with Mo and Mn. Whereas SS316L is a conventional alloy, Nitinol is a less-utilized alloy which has gained popularity in the field of biomaterials due to its shape memory property and corrosion resistance.45–47 Modifying these substrates has been proven to be challenging perhaps because only some of the surface species in the alloy react with the organic. On other metal oxide surfaces incomplete film coverage has been attributed to many parameters including low hydroxyl content, surface roughness48, 49 and surface defects49.
In this study, monolayer formation on the alloy oxide surface of SS316L and Nitinol was compared to monolayer formation on the constituent metal oxide surfaces, iron, nickel, titanium, chromium, molybdenum and manganese by long chain aliphatic acids with various head groups (sulfonic acid, phosphonic acid, hydroxamic acid and carboxylic acid). The extent of monolayer formation in these systems was studied by diffuse reflectance infrared Fourier transform spectroscopy (DRIFT), Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS), contact angle goniometry and atomic force microscopy (AFM), in order to elucidate the role of constituent metals and head group in monolayer formation on alloys.
Experimental
Materials
Stainless Steel foils (SS316L, 99.99% pure), molybdenum foils (99.9% pure), iron foils (99.5% pure), nickel foils (>99% pure) and titanium foils (>99% pure) of 0.25mm thickness were obtained from Goodfellow Inc. Chromium pieces of 1mm–6mm size (99.998% pure) and manganese pieces of 3mm–12mm size (99.99% pure) were obtained from Kurt J. Lesker Company. Nitinol foils (NiTi, 51% nickel: 49 % titanium; 0.008” thickness, >99.0 % pure) were purchased from Johnson Matthey, Inc. Tetrahydrofuran (Optima grade), methanol, octadecylcarboxylic acid (ODCA, 98+ %) were obtained from Aldrich Chemical Co. Tetrahydrofuran (THF) was distilled over sodium and stored under nitrogen. Octadecylphosphonic acid (ODPA, 98%) was obtained from Alfa Aesar. Octadecylhydroxamic acid (ODHA) was synthesized in the laboratory as previously reported.16 Sodium 1-octadecanesulfonate (97%) was obtained from Fluka and acidified using hydrochloric acid to form the corresponding acid. The octadecylsulfonic acid (ODSA) formed was then analyzed by IR, mass spectrometry and NMR. Hydrochloric acid (ACS certified Plus) was purchased from Fisher Scientific and used without further purification.
Equipment
All diffuse reflectance infrared spectroscopy (DRIFT) measurements were performed on a Nexus 470 FTIR equipped with a diffuse reflectance attachment from Thermo Electron Corporation. Contact angle measurements were obtained on a VCA Optima Goniometer. Atomic force microscope (AFM) images were taken on a PicoSPM from Molecular Imaging Corporation (Agilent Technologies). Mass spectra were collected on a high resolution atmospheric pressure time-of-flight mass spectrometer purchased from Agilent Technologies
Substrate preparation and monolayer formation
Stainless steel 316L (SS316L), Nitinol (NiTi), iron, nickel, titanium and molybdenum substrates were cut into 1 cm × 1 cm coupons and sanded using 220, 320, 400 and 600 grit silicon carbide papers. Manganese and chromium pieces were ingots and therefore not sanded because of their morphology. All substrates were cleaned by ultra-sonication in methanol (15 min) followed by immersion in boiling methanol to remove organic residue and metallic dust. The cleaned substrates were stored in an oven at 100°C.
Films were formed on the native oxide surface of the two alloys and their constituents using solution deposition methods (either dipping or spraying).17, 32, 36, 37 The experimental procedure used to form monolayers for each of the metal /alloy- acid combination is tabulated in Table 1. The final procedures were arrived at by varying experimental procedures including increasing the deposition time, organic concentration, deposition temperature, and solution deposition method in an attempt to obtain successful film formation. Numerous unsuccessful procedures were performed and not included in the manuscript. The procedures adopted to form monolayers are as discussed below. All substrates were stored at 100°C prior to deposition.
The substrates were sprayed using a 0.35mM solution of the acid at room temperature and allowed to dry at room temperature.
The substrates were sprayed using a 0.75mM solution of the acid at room temperature and allowed to dry at room temperature for 20 minutes and then the same spraying procedure was repeated one more time.
A 1.5mM solution was used to spray the substrates 3 times at room temperature and dried in the oven at 120°C for 20 minutes between sprays.
Substrates were dipped in 1mM solution of the acid at room temperature for 1 minute.
Substrates were left in a 1mM solution of the organic acid for 3–5 minutes at room temperature.
Substrates were dipped in a warm (50°C) 1mM solution of the acid for 2 hours and then dried in the oven at 100°C overnight.
Substrates were placed on a dish which was then placed in an ice bath for 1 hour. The substrates were then dipped in a warm (50°C) 1mM solution of the acid for 2 hours, taken out of solution without solvent meniscus and stored in the oven at 100°C overnight.
Substrates were placed on a dish which was then placed in an ice bath for 1 hour. The substrates were then dipped in a warm (50°C) 2 mM solution of the acid for 2 hours, taken out of solution without solvent meniscus and stored in the oven at 100°C overnight.
Substrates were placed on a dish and the dish was placed in an ice bath for 1 hour. The substrates were then dipped in a warm (50°C) 2 mM solution of the acid for 2 hours, taken out of solution with solvent meniscus and stored in the oven at 100°C overnight.
Table 1.
Procedure numbers used for each acid/ metal combination.
| ACIDS | Sulfonic Acid |
Phosphonic acid |
Hydroxamic acid |
Carboxylic acid |
|---|---|---|---|---|
| Metals | ||||
| SS316L | 6 | 4 | 9 | 7 |
| Nitinol | 2 | 1 | 3 | 3 |
| Nickel | 2 | 1 | 9 | 8 |
| Titanium | 2 | 1 | 3 | 3 |
| Iron | 6 | 4 | 9 | 8 |
| Chromium | 6 | 4 | 9 | 8 |
| Molybdenum | 6 | 5 | 9 | 8 |
| Manganese | 6 | 4 | 9 | 8 |
The monolayers were checked for ordering of the alkyl chains by IR and contact angle to confirm condensed film formation. Contact angle data and AFM imaging of dipping and spraying procedures showed similar organization of the films on the surface.
Characterization of the monolayers
Infrared Spectroscopy
After the formation of monolayers, DRIFT was used to analyze the ordering and orientation of the molecules in the monolayer formed. Spectra were collected under nitrogen for 1024 scans with a resolution of 1 cm−1 on each sample and ratioed with a background reference spectrum of the same metal or alloy. After verifying the presence of films after the deposition, the samples were rinsed in THF for 15 mins and analyzed by DRIFT spectroscopy again to confirm the presence of the films. The substrates were then sonicated in THF for 15 mins to test the mechanical stability of the films.
Contact Angle Measurement
Static contact angle of water on the films was measured to determine the wettability of the surface. The average contact angle value and standard deviation of six measurements each on at least three samples (eighteen measurements total) has been reported.
Atomic Force Microscopy
An atomic force microscope (AFM) operating in non-contact mode at ambient conditions was used to confirm the uniformity of the film on the surface. The cantilever tip with a resonance frequency of 160–170 kHz and a typical spring constant of 40 N/m were obtained from Agilent-Molecular Imaging Corp.
Mass Spectrometry
A high resolution MALDI-TOF MS purchased from Agilent Technologies. with pulsed dynamic focusing was used to characterize the molecules on the substrate. MS analyses of the ions were detected in positive mode. The matrix, α-cyano-4-hydroxycinnamic acid (CHCA) (Sigma-Aldrich, >99.0% purity), was used without further purification and added in THF (10mg/mL). Samples for MALDI MS analysis were prepared by “dried-drop method”. A one microliter aliquot of the matrix solution was placed on each of the modified substrates and then dried at room temperature. The samples were then placed directly onto a MALDI sample plate using double sided tape and loaded into the MALDI-TOF. Analyte ionization was achieved by focusing a pulse of a laser light onto the sample/matrix preparation.
Results
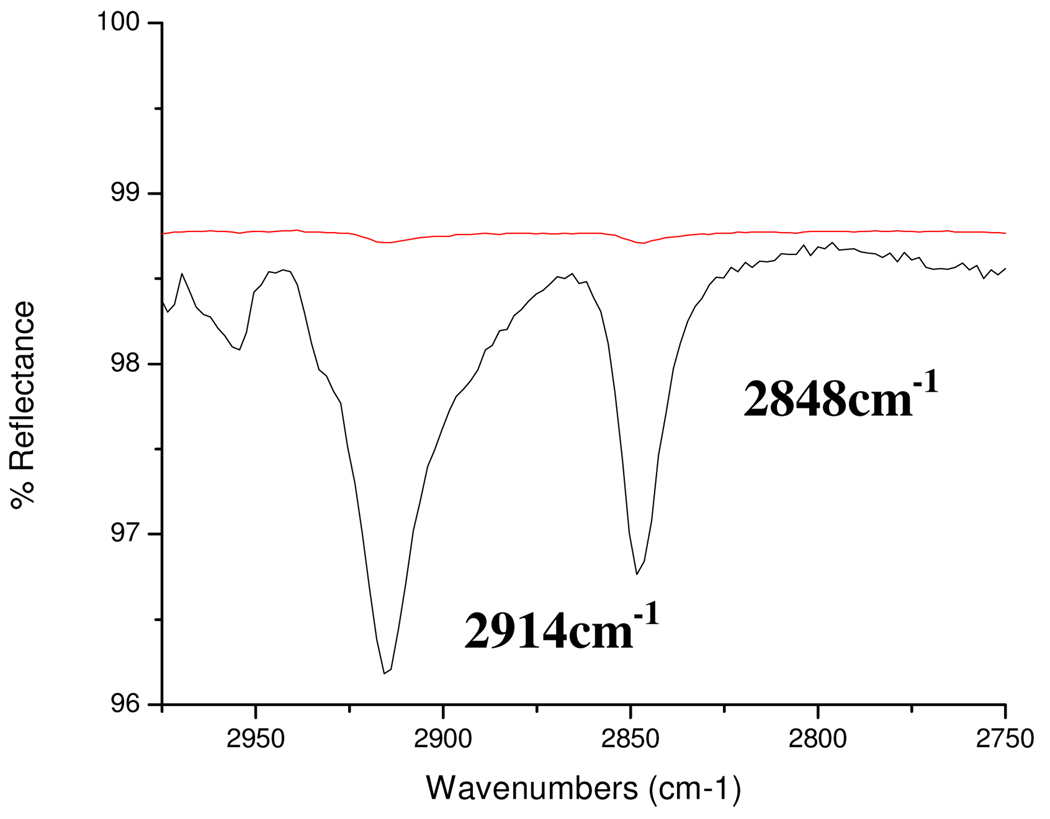
Self assembled monolayers were formed on the native oxide surface of Stainless steel 316L and Nitinol alloys. X-ray photoelectron spectroscopy (XPS) analysis of the stainless steel 316L substrate was performed previously using a Phoibos 150 hemispherical energy analyzer and a monochromatized Al (1486.6 eV) source. Compositional results for the SS316L reference substrate were in reasonable agreement with the nominal SS316L bulk elemental composition50 (Surface composition: Fe 66.01%, Cr 19.19 %, Ni 9.17 %, Mn 3.22% and Mo 2.42%.). The Nitinol substrate’s surface composition was 50% each of nickel and titanium oxides.45, 51 Thus, iron, chromium, nickel, manganese, molybdenum and titanium were used as the constituent metals for the alloys in this study. A long carbon chain of 18 was utilized as the standard to optimize van der Waals interactions and to prevent chain length variation from affecting the formation of ordered self assembled monolayers. The films formed were characterized by DRIFT spectroscopy after deposition, rinse in THF for 15 minutes and after sonication in THF for 15 minutes. In the spectra of the modified substrates, the C–H stretches of the methylene group are used as the reference peaks for alkyl chain organization in the SAM.13, 52, 53 A spectra which contained νCH2 asym≤ 2918 cm−1 and νCH2 symm≤ 2848 cm−1 indicated that the alkyl chains in the film were ordered with the molecules organized in all-trans configuration on the substrate (Figure 1). The position of the peaks corresponding to νCH2 asym after rinsing with THF and after sonication in THF are provided in Table 2 for the acid- metal oxide and acid- alloy oxide systems. If the organic molecules were removed by rinse or sonication, the data is represented by dotted lines (----).
Figure 1.
DRIFT spectra of the C–H stretching region of octadeylsulfonic acid film on Mn after rinse (bottom) and sonication (top). The film is removed by sonication. The νCH2 asym= 2914 cm−1 and νCH2 symm= 2848 cm−1.
Table 2.
CH2 asymm stretching frequency for each oxide-acid combination in the study. Rinse= Rinse in THF; Sonic= Sonication in THF.
| Sulfonic acid νCH2 assym (cm−1) |
Phosphonic acid νCH2 assym (cm−1) |
Hydroxamic acid νCH2 assym (cm−1) |
Carboxylic acid νCH2 assym (cm−1) |
|||||
|---|---|---|---|---|---|---|---|---|
| Rinse | Sonic | Rinse | Sonic | Rinse | Sonic | Rinse | Sonic | |
| SS 316L | 2913 | 2916 | 2912 | 2911 | 2916 | ---- | 2915 | 2917 |
| Nitinol | 2915 | ---- | 2914 | 2913 | 2915 | ---- | ---- | ---- |
| Nickel | 2918 | ---- | 2914 | 2915 | 2916 | ---- | ---- | ---- |
| Titanium | 2916 | ---- | 2913 | 2913 | 2915 | ---- | ---- | ---- |
| Iron | 2916 | ---- | 2915 | 2916 | ---- | ---- | 2915 | 2915 |
| Chromium | 2915 | ---- | 2914 | 2914 | ---- | ---- | ---- | ---- |
| Molybdenum | 2914 | ---- | 2914 | 2914 | 2913 | 2913 | ---- | ---- |
| Manganese | 2914 | ---- | 2914 | 2914 | 2914 | ---- | ---- | ---- |
From Table 2, it is clear that phosphonic acid forms the most stable interaction with all of the substrates as the films remain intact after sonication on the metals and alloys. Sulfonic acid forms films on all of the surfaces and is stable to rinsing in solvent but only remains on SS316L after sonication. Carboxylic acid only formed films on SS316L and its major component Fe oxide and the films were stable on both surfaces.
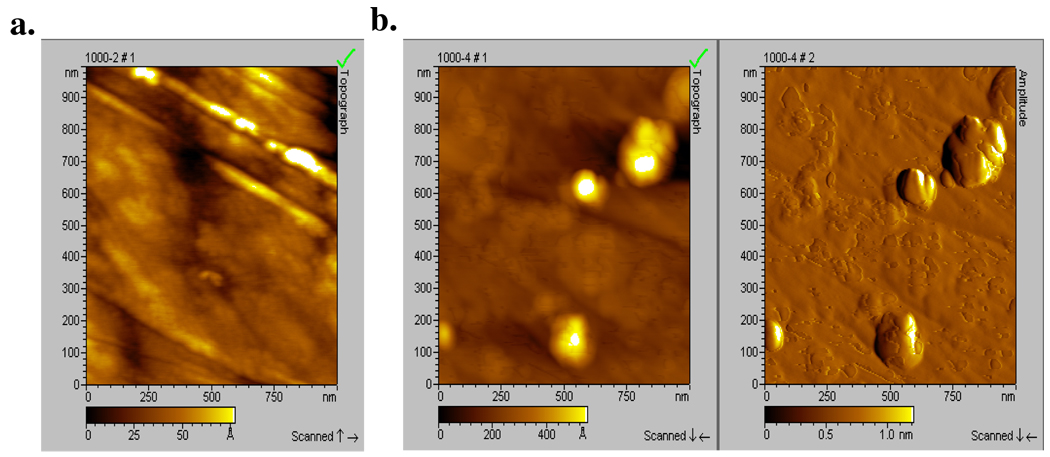
Hydroxamic acid formed ordered films on more substrates than carboxylic acid. Previous reports on hydroxamic acid films indicated that they were more stable than carboxylic or phosphonic acid on metal oxides such as copper, aluminum and iron16 and had good corrosion inhibition efficiency due to their well-ordered alkyl chains and film formation.41, 54, 55 In this study, the hydroxamic acid films formed were stable to rinse but not to sonication, which was not performed in the previous report. To understand this result, AFM images of nitinol before and after the deposition and rinsing of the octadecylhydroxamic acid (ODHA) film is shown in Figure 2. The rms roughness of the control and modified substrates were compared. Modified surfaces with a similar rms roughness to the control surface are considered to be uniform films that follow the contour of the surface, while modified surfaces that have a much larger rms roughness than the control are multilayer or non-uniform films.36, 56 The rms roughness of the control and modified substrate were 14 Å and 75 Å, respectively (Figure 2). These values and the images clearly indicate that the surface is non-uniformly covered by multilayers and islands of agglomerates. These images are correlated to the contact angle values obtained of ODHA on nitinol which are lower than those expected for a hydrophobic surface as shown in Table 3. Moreover, the film was completely removed after sonication, and so it is concluded that the hydroxamic acid films are weakly bound, perhaps due in part to micelle formation seen in the images.
Figure 2.
(a) AFM topography image of clean NiTi (rms roughness=14 Å), (b) AFM image of NiTi surface after modification with ODHA and rinsed with THF (rms roughness=75Å) in topography and amplitude
Table 3.
Static contact angle data for rinsed organic films on metals and alloy oxides
| ACIDS | Sulfonic acid |
Phosphonic acid |
Hydroxamic acid |
Carboxylic acid |
||||
|---|---|---|---|---|---|---|---|---|
| Oxide | θ ° | ± Stdev |
θ ° | ± Stdev |
θ ° | ± Stdev |
θ ° | ± Stdev |
| SS 316L | 90 | 4 | 108 | 3 | 76 | 9 | 104 | 1 |
| Nitinol | 89 | 8 | 107 | 3 | 103 | 3 | ---- | ---- |
| Nickel | 93 | 8 | 109 | 5 | 100 | 4 | ---- | ---- |
| Titanium | 82 | 18 | 96 | 5 | 101 | 3 | ---- | ---- |
| Iron | 83 | 10 | 98 | 4 | ---- | ---- | 87 | 5 |
The films stable to rinse on the alloys and the major components of the alloy were further characterized by contact angle goniometry (Table 3). Contact angles can be a useful indicator of film quality and wettability of the surface when used in conjunction with other data. Contact angle values for the unmodified substrates were in the range of 50–60°. (Specific values are in the supplemental information). Here, the contact angles for phosphonic acid SAMs were the most hydrophobic. Sulfonic acid films produced lower contact angle values suggesting heterogeneous or incomplete surface films as can be seen in the AFM images of these substrates (AFM in supplemental information). For hydroxamic acid films on SS316L the standard deviations were high indicating that the films were not uniform on this surface. Contact angle values for films on nitinol, nickel and titanium were consistent with hydrophobic uniform films. Finally, carboxylic acid formed monolayers only on SS316L and iron and presented a hydrophobic interface on both of them indicating that the methyl tail group is presented at the interface.
The interaction between the organic moiety and the surface oxide can be characterized by DRIFT spectroscopy. This interaction can be either mono-, bi-, or tri-dentate and cases of each have been reported in the literature (Figure 3)17, 36, 37, 57.
Figure 3.
Monodentate, bidentate and tridentate bonding of phosphonic acid on stainless steel 316L.
The bonding mode of the acid with the metals and alloys after sonication has been tabulated in Table 4 based on IR spectroscopy. For phosphonic acid SAMs the IR stretch values in the P-O region, which corresponds to 1300-950 cm−1, provides valuable information about the type of bonding of the head group with the substrate. IR bands at 1227 cm−1 and 950 cm−1 are assigned to ν P=O and ν P-OH respectively and the peaks at 1074 cm−1and 1010 cm−1 are assigned to the symmetric and asymmetric stretch of νPO3.19, 20, 40 If the peak assigned to P-O and P=O stretches are observed, coupled with the disappearance of ν P-O-H = 920 cm−1, a bidentate coordination mode of the molecule to the surface is indicated.37 This bonding mode was observed for stainless steel, nickel, titanium and iron oxides after each rinse and adhesion test. Some spectra of the modified iron substrates, however, were consistent with bidentate bonding modes while others were consistent with tridentate bonding. This is consistent with phosphonic acid SAMs on silicon oxide, where binding is tridentate.58 The presence of νP=O, νP-O and shifted νP-O-H indicates monodentate bonding36 as observed for nitinol, molybdenum and manganese.
Table 4.
Proposed bonding motif of organic films after sonication; monodentate, bidentate, tridentate or hydrogen bonding with substrate.
| ACIDS | Sulfonic acid |
Phosphonic acid |
Hydroxamic acid |
Carboxylic acid |
|---|---|---|---|---|
| Metals | ||||
| SS316L | Mono | Bi | ---- | Bi |
| Nitinol | ---- | Mono | ---- | ---- |
| Nickel | ---- | Bi | ---- | ---- |
| Titanium | ---- | Bi | ---- | ---- |
| Iron | ---- | Bi/ Tri | ----- | Mono |
| Chromium | ---- | Mono or H | ----- | ---- |
| Molybdenum | ----- | Mono | Bi | ---- |
| Manganese | ---- | Mono | ---- | ----- |
For carboxylic acids, the interaction of the head group with the substrate is correlated to the COO− stretch frequency in infrared spectroscopy.59 The IR spectrum of solid octadecylcarboxylic acid contains νC=O at 1700 cm−1 and νC–O at 1465 cm−1.60 Spectra of bound carboxylic acid with an absence of the carbonyl stretch (νC=O) and the presence of the νCOO−asymm peak around 1510 cm−1 and the νCOO−symm peak around 1400 cm−1 implies that the head group bonds to the surface through two oxygen atoms. This type of bidentate bonding is observed in silver or copper adlayers on gold61 and on air-exposed silver surfaces.62 For SAMs on aluminum and stainless steel, multiple carboxylate species have been observed.13, 34, 43 Here, a bidentate bonding of carboxylic acid with stainless steel was observed32 while the interaction on iron oxide was a mixture of monodentate and bidentate (νC=O = 1656 cm−1 and νCOO−symm, νCOO−asymm peak at 1470, 1580 cm−1, respectively).
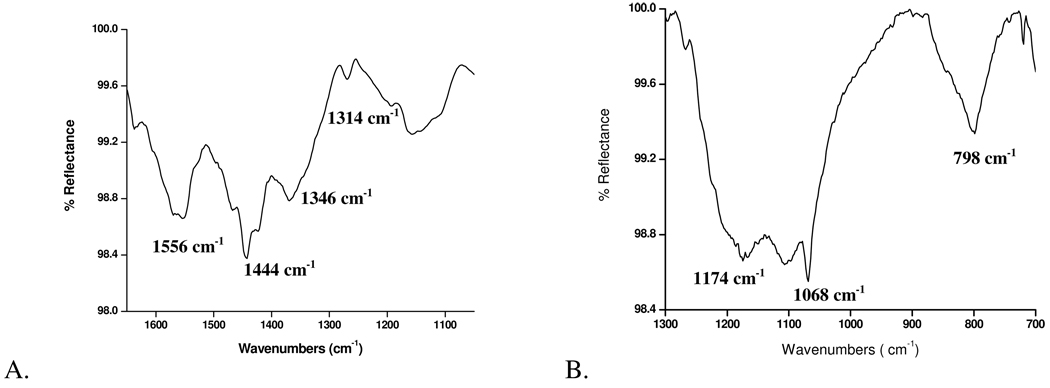
The nature of the interaction between the hydroxamic acid molecule and the surface can be determined from the shifts and broadening of νC=O, νN–O, and νN-H, indicating a change in head group bonding.16, 136 The CO and NH region of the ODHA solid contains νN-H = 3252 and 1565 cm−1, νC=O = 1660 cm−1, and νN–O = 1080 cm−1. The appearance of peak assigned to νC–O and the absence of νC=O peak coupled with a shift to lower frequency of the peak assigned to νN-H and νN–O, represents a bidentate bonding with the substrate as observed on molybdenum samples (Figure 4A).
Figure 4.
A. IR spectra of molybdenum modified with ODHA showing bidentate bonding with peaks centered at 1314 (νCO), 1444 and 1556 cm−1 (νCOO−symm, νCOO−asymm respectively) and 1346 cm-1 for(νC–N). B. IR spectrum of SS316L modified with ODSA with peaks centered at νSOasym= 1174 cm−1, νSOsym = 1068 cm−1 and νC–S= 798 cm−1.
For sulfonic acid, the SO region of the ODSA solid shows νSOasym= 1178 cm−1, νSOasym = 1059 cm−1, νSOH= 946 cm−1 and νC–S= 794 cm−1. After deposition, rinsing and sonication on SS316L, the ODSA remains and the binding region indicates that there is a strong interaction between ODSA and SS316L due to the disappearance of the peak attributable to νSOH (946 cm−1; Figure 4B) and the broadening of the peaks attributable to S-O stretching indicating a bonding interaction between the oxygens and the surface.40, 63 Due to the changes in the spectra and the fact that the monolayer was not removed by rinse and sonication, a monodentate bonding interaction (and not a weaker hydrogen-bonding interaction) with SS316L is proposed.
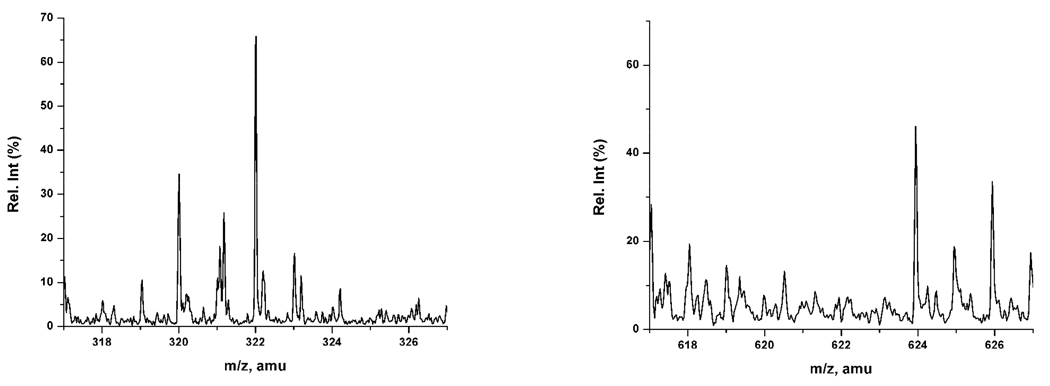
To determine if the films remaining after sonication were monolayers or multilayers MALDI spectrometry was performed. Previous work in our group has shown that for monolayer films a peak for the mass of the sodiated monomer is seen in the mass spectrum and dimer peaks are not observed 36 (Figure 5). In the current study, for all films that were stable to sonication only the sodiated monomer peaks were observed in the mass spectrum. This indicates that the stable films were all monolayers.
Figure 5.
MALDI spectra of monolayer film of octadecylhydroxamic acid sodiated on titanium m/z 322.017 amu (ODHA + Na+). Dimer peak is not observed at 621 amu.
Discussion
Other reports have postulated many possible reasons for the differences in film formation on metal oxides. The theories rely on a combination of organic and surface parameters such as oxide content of the alloy, organic moiety pKa, basicity of the surface,14 and hydroxyl content.14, 16, 35, 38, 64 In the two alloys studied here, the surfaces are composed of the oxides of the metals in similar ratios to the bulk alloy.4, 32, 37, 50, 65 Film formation, uniformity and stability on the surface could potentially be controlled by the most abundant metal oxide, the most reactive or the least reactive metal in the alloy. The results tabulated above do not pinpoint a controlling factor based on alloy content but clearly show that the presence of non-reactive metal oxides on the alloy surface will not prevent the formation of uniform, stable films, as in the case of SS316L.
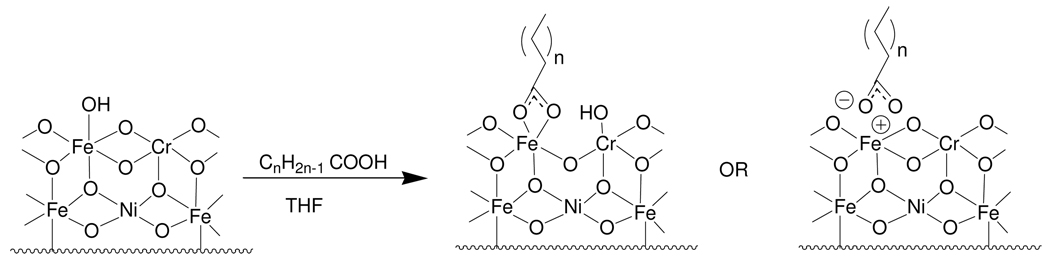
For oxide substrates, formation of SAMs is often considered to be due to acid-base chemistry that occurs between the acid and the metal oxide substrate. In one proposed mechanism, the oxide, in the form of μ- oxo and hydroxyl groups, act as Lewis bases and are thus reactive under acidic conditions.13, 66 This reaction leads to the formation of a strong covalent bond (Figure 6).13 An alternate mechanism is that proton transfer from the organic to the surface hydroxyls followed by dehydration leads to an ionic interaction between the deprotonated organic and the dehydrated surface (Figure 6).13
Figure 6.
Proposed binding schemes for organic acids on metal oxide surfaces. Left: Covalent Right: Ionic using carboxylic acids on SS316L.
A notable difference between the acids under investigation was their head group acidity. The pKa of sulfonic acid is approximately 2,67 making it the strongest acid in this study, while phosphonic acids, have a pKa of 4.5.68, 69 Two different surface pKa values are reported for the carboxylic acid deprotonation: a pKa value of 5 corresponds to COOH groups hydrogen-bonded to the interfacial water only, while a pKa of 9.5 corresponds to COOH headgroups hydrogen-bonded to each other as seen in organic solvent solutions.70 In this study, the carboxylic acid is dissolved in THF. Hydroxamic acid (pKa = 9)16, 71–73 is stabilized by the resonance between the carbonyl and the hydroxyl group and therefore, hydroxamic acids tend to be more acidic than carboxylic acids.72 Thus, the pKa values of the head groups utilized in this study in increasing order is: sulfonic acid > phosphonic acid > hydroxamic acid > carboxylic acid. Therefore, if low organic molecule pKa facilitates film formation through an acid-base reaction, sulfonic acids should form films more easily on metal oxides than carboxylic acids.18, 31, 74–76
Based on our observations, the ability to form films on the alloys and their substituents can be summarized as follows: Sulfonic acid = Phosphonic acid > Hydroxamic acid > Carboxylic acid. Therefore, films did form on the surface based on organic pKa. However, stability of the films was not strictly related to pKa. The most stable films on the largest number of substrates are formed by phosphonic acids. This is in agreement with literature data that phosphonic acids bind more strongly to metal oxides than other acids.18, 31, 74–76
The proposed mechanisms in Figure 6 are a result of reports that metal oxides with higher hydroxyl contents, such as aluminum oxide, react with weaker acids such as carboxylic acids,13, 14, 16, 35, 77 while lower hydroxyl content surfaces, such as titanium dioxide, are considered to be less reactive, and therefore only react with very acidic molecules such as phosphonic acids.38, 78–81 The number of hydroxyl groups on the surface of the metal could affect the film formation by behaving as anchoring sites for the acid.14, 16, 35, 38, 64 Conversely, Lu et al observed that for Ti oxide surface, the formation of SAMs was not affected by surface hydroxyls.82 Based on literature reports, the effect of hydroxyl content on surface-organic interactions was analyzed (Table 5).16, 82–85 (The hydroxyl contents for the alloys were not reported in the literature in OH/nm2. 82, 86–88) The hydroxyl content for the metal oxides were similar, except for molybdenum and titanium, which are lower. Although, the molybdenum oxide surface hydroxyl content was significantly lower than the others, films formed on this surface in the same manner as the other oxides and better than on nickel oxide. A range of values are reported for the hydroxyl content of titanium oxide and it only stably reacts with phosponic acid. The hydroxyl contents for the alloy and metal systems in decreasing order are: Fe > Mn > Ni > Cr > Ti > Mo. This data does not predict the ability of the surfaces to form stable films with organic acids.
Table 5.
Isoelectric points reported previously and hydroxyl content for the metals and alloys
| Metals | Isoelectric point | Hydroxyl content (OH / nm2) |
|---|---|---|
| SS 316L | 5.8, 8.5 | − |
| Nitinol | Not reported | − |
| Nickel | 11 | 14–15 |
| Titanium | 4–6 | 7–12.5 |
| Iron | 7 | 15 |
| Chromium | 6.7 | 13 |
| Molybdenum | 2.5 | 2.4–2.7 |
| Manganese | 4 | 14.5 |
Previously, researchers correlated the reactivity of surfaces and organics with the isoelectric point (IEP) of the metal. 16, 20, 89 Under this theory the pKa of the organic acid has to be lower than the IEP of the metal to be capable of forming ordered films on the metal oxide surfaces. Therefore, higher isoelectric points led to more reactive surfaces towards the acid, thus resulting in a stronger coordination when bound to the surface.14 Tao et al. observed that the formation of SAMs on silver, copper and aluminum oxide surfaces depended on the isoelectric point of the metal.14 The IEP of titanium (4–6), molybdenum (2.5), manganese(4), chromium(6.7), iron(7), and nickel(11) have been tabulated from literature reports in Table 5.83, 84, 90–92 The IEP of SS316L was previously calculated by XPS and reported to be 5.8 whereas a more recent work using potentiometric titrations by Takahasi93 reported the IEP of stainless steel 316L to be 8.5. The IEP of nitinol alloy is unknown because one would need a salt of the alloy, which is not available.83, 84
Utilizing the idea that the IEP was a major controlling factor in film formation, sulfonic and phosphonic acid are the only acids capable of forming strong and stable interactions on iron, chromium, manganese, SS316L and titanium oxide surface where as the nickel oxide surface can theoretically be modified using carboxylic, hydroxamic, sulfonic and/or phosphonic acids. In contrast, our observations show that phosphonic acid reacts with all the metals and alloys and remains attached even after sonication as predicted. However, while sulfonic acid interacts with most oxides the interaction is not strong and is removed by sonication. Carboxylic acid reacts stably with iron and stainless steel oxides to form ordered films but not with nickel oxide which was predicted by the IEP rule. This data indicates that the IEP of a surface may be a good predictor for the formation of films on surfaces, but it is not the controlling factor and other parameters play a role.
Conclusions
From this study, it is clear that film formation on alloy oxides is related to several factors, including the reactivity of the metals that compose the surface. However, the nonreactive components of the alloy do not prevent complete film formation. Therefore, the reactive metal sites may act as nucleation sites for film formation on the alloys. For both alloys and their constituent metals, decreasing pKa of the organic moiety and a higher isoelectric point of the surface generally leads to film formation. However, the film stability is not necessarily related to these factors. Further experiments into the role of these and other parameters such as surface roughness, grain boundaries in alloys, electron affinity and ionization potential by both experimental and computational studies are needed.
Supplementary Material
Acknowledgments
We thank Dr. Toby Chapman (University of Pittsburgh) for use of the contact angle meter and Dr. Stephanie Wetzel (Duquesne University) for help in using the MALDI-TOF MS. Additionally, the authors thank the Pennsylvania Department of Health CURE Program, the ACS-PRF and its donors and the National Institutes of Health (NIAMS 1 R15 AR056864-01) for generous funding of this project. RE thanks the NSF-REU DOD-ASSURE program for summer funding.
Footnotes
Supporting Information Available: Additional MALDI-TOF, AFM and contact angle data is available in supporting information as noted in the text. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Rozsnyai LF, Wrighton MS. Chem Mater. 1996;8(2) 309-&. [Google Scholar]
- 2.Nozawa K, Nishihara H, Aramaki K. Corrosion Science. 1997;39(9):1625–1639. [Google Scholar]
- 3.Kovaric BC, Kokona B, Schwab AD, Twomey MA, de Paula JC, Fairman R. J Am Chem Soc. 2006;128(13):4166–4167. doi: 10.1021/ja056357q. [DOI] [PubMed] [Google Scholar]
- 4.Wang DA, Feng LX, Ji J, Sun YH, Zheng XX, Elisseeff JH. J Biomed Mater Res A. 2003;65A(4):498–510. doi: 10.1002/jbm.a.10533. [DOI] [PubMed] [Google Scholar]
- 5.Svedhem S, Hollander CA, Shi J, Konradsson P, Liedberg B, Svensson SCT. J Org Chem. 2001;66(13):4494–4503. doi: 10.1021/jo0012290. [DOI] [PubMed] [Google Scholar]
- 6.Jiang YG, Wang ZQ, Yu X, Shi F, Xu HP, Zhang X. Langmuir. 2005;21(5):1986–1990. doi: 10.1021/la047491b. [DOI] [PubMed] [Google Scholar]
- 7.Lummerstorfer T, Hoffmann H. J Phys Chem B. 2004;108(13):3963–3966. [Google Scholar]
- 8.Park BS, Heo SJ, Kim CS, Oh J-E, Kim J-M, Lee G, Park WH, Chung C-P, Min B-M. J. Biomed. Mat. Res. Part A. 2005;74A(4):640–651. doi: 10.1002/jbm.a.30326. [DOI] [PubMed] [Google Scholar]
- 9.Cox JD, Curry MS, Skirboll SK, Gourley PL, Sasaki DY. Biomaterials. 2002;23(3):929–935. doi: 10.1016/s0142-9612(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 10.Fan FRF, Yao YX, Cai LT, Cheng L, Tour JM, Bard AJ. J Am Chem Soc. 2004;126(12):4035–4042. doi: 10.1021/ja0359815. [DOI] [PubMed] [Google Scholar]
- 11.Nitahara S, Akiyama T, Inoue S, Yamada S. J Phys Chem B. 2005;109(9):3944–3948. doi: 10.1021/jp046776u. [DOI] [PubMed] [Google Scholar]
- 12.Collman JP, Devaraj NK, Chidsey CED. Langmuir. 2004;20(4):1051–1053. doi: 10.1021/la0362977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allara DL, Nuzzo RG. Langmuir. 1985;1:45–52. [Google Scholar]
- 14.Tao Y. J. Pys. Chem. 1993;115:4350–4358. [Google Scholar]
- 15.Tao YT, Hietpas GD, Allara DL. J Am Chem Soc. 1996;118(28):6724–6735. [Google Scholar]
- 16.Folkers JP, Gorman CB, Laibinis PE, Buchholz S, Whitesides GM, Nuzzo RG. Langmuir. 1995;11(3):813–824. [Google Scholar]
- 17.Quinones R, Raman A, Gawalt ES. Thin Solid Films. 2008;516(23):8774–8781. [Google Scholar]
- 18.Pawsey S, Yach K, Reven L. Langmuir. 2002;18(13):5205–5212. [Google Scholar]
- 19.Gawalt ES, Lu G, Bernasek SL, Schwartz J. Langmuir. 1999;15(26):8929–8933. [Google Scholar]
- 20.Gao W, Dickinson L, Grozinger C, Morin FG, Reven L. Langmuir. 1996;12(26):6429–6435. [Google Scholar]
- 21.Palma R, Laureyn W, Frederix F, Bonroy K, Pireaux JJ, Borghs G, Maes G. Langmuir. 2007;23(2):443–451. doi: 10.1021/la061951e. [DOI] [PubMed] [Google Scholar]
- 22.Miller JB, Schwartz J, Bernasek SL. J. Am. Chem. Soc. 1993;115(18):8239–8247. [Google Scholar]
- 23.Midwood KS, Carolus MD, Danahy MP, Schwarzbauer JE, Schwartz J. Langmuir. 2004;20(13):5501–5505. doi: 10.1021/la049506b. [DOI] [PubMed] [Google Scholar]
- 24.Diez-Perez I, Luna M, Teheran F, Ogletree DF, Sanz F, Salmeron M. Langmuir. 2004;20(4):1284–1290. doi: 10.1021/la030336x. [DOI] [PubMed] [Google Scholar]
- 25.Volmer-Uebing M, Stratmann M. Applied Surface Science. 1992;55(1):19–35. [Google Scholar]
- 26.Hild R, David C, Muller HU, Volkel B, Kayser DR, Grunze M. Langmuir. 1998;14(2):342–346. [Google Scholar]
- 27.Sinapi F, Naji A, Delhalle J, Mekhalif Z. Surf Interface Anal. 2004;36(11):1484–1490. [Google Scholar]
- 28.Duwez AS, Jonas U, Klein H. Chemphyschem. 2003;4(10):1107–1111. doi: 10.1002/cphc.200300743. [DOI] [PubMed] [Google Scholar]
- 29.Mekhalif ZRJ, Pireaux J-J, Delhalle J. Langmuir. 1997;13(8):2285–2290. [Google Scholar]
- 30.Sung MM, Sung K, Kim CG, Lee SS, Kim Y. J. Phys. Chem. B. 2000;104(10):2273–2277. [Google Scholar]
- 31.Pawsey S, Yach K, Halla J, Reven L. Langmuir. 2000;16(7):3294–3303. [Google Scholar]
- 32.Raman A, Gawalt ES. Langmuir. 2007;23(5):2284–2288. doi: 10.1021/la063089g. [DOI] [PubMed] [Google Scholar]
- 33.van den Brand J, Blajiev O, Beentjes PCJ, Terryn H, de Wit JHW. Langmuir. 2004;20(15):6308–6317. doi: 10.1021/la0496845. [DOI] [PubMed] [Google Scholar]
- 34.Lim MS, Feng K, Chen XQ, Wu NQ, Raman A, Nightingale J, Gawalt ES, Korakakis D, Hornak LA, Timperman AT. Langmuir. 2007;23(5):2444–2452. doi: 10.1021/la061914n. [DOI] [PubMed] [Google Scholar]
- 35.Sondag AHM, Raas MC. J. Chem. Phys. 1989;91:4926–4931. [Google Scholar]
- 36.Quinones R, Raman A, Gawalt ES. Surf Interface Anal. 2007;39(7):593–600. [Google Scholar]
- 37.Raman A, Dubey M, Gouzman I, Gawalt ES. Langmuir. 2006;22(15):6469–6472. doi: 10.1021/la060636p. [DOI] [PubMed] [Google Scholar]
- 38.Gawalt ES, Avaltroni MJ, Koch N, Schwartz J. Langmuir. 2001;17(19):5736–5738. [Google Scholar]
- 39.Yee C, Kataby G, Ulman A, Prozorov T, White H, King A, Rafailovich M, Sokolov J, Gedanken A. Langmuir. 1999;15(21):7111–7115. [Google Scholar]
- 40.Fiurasek P, Reven L. Langmuir. 2007;23(5):2857–2866. doi: 10.1021/la0629781. [DOI] [PubMed] [Google Scholar]
- 41.Telegdi J, Rigo T, Kalman E. Journal of Electroanalytical Chemistry. 2005;582(1–2):191–201. [Google Scholar]
- 42.Shustak G, Shaulov Y, Domb AJ, Mandler D. Chemistry-a European Journal. 2007;13(22):6402–6407. doi: 10.1002/chem.200601559. [DOI] [PubMed] [Google Scholar]
- 43.Shustak G, Domb AJ, Mandler D. Langmuir. 2004;20(18):7499–7506. doi: 10.1021/la036470z. [DOI] [PubMed] [Google Scholar]
- 44.Mahapatro A, Johnson DM, Patel DN, Feldman MD, Ayon AA, Agrawal CM. Langmuir. 2006;22(3):901–905. doi: 10.1021/la052817h. [DOI] [PubMed] [Google Scholar]
- 45.Shevchenko N, Pham MT, Maitz MF. Applied Surface Science. 2004;235(1–2):126–131. [Google Scholar]
- 46.Shabalovskaya SA. Bio-Medical Materials and Engineering. 2002;12(1):69–109. [PubMed] [Google Scholar]
- 47.Kapanen A, Ryhänen J, Danilov A, Tuukkanen J. Biomaterials. 2001;22(18):2475–2480. doi: 10.1016/s0142-9612(00)00435-x. [DOI] [PubMed] [Google Scholar]
- 48.Leopold MC, Bowden EF. Langmuir. 2002;18(6):2239–2245. [Google Scholar]
- 49.Priebe A, Pucci A, Otto A. J Phys Chem B. 2006;110(4):1673–1679. doi: 10.1021/jp054803q. [DOI] [PubMed] [Google Scholar]
- 50.Wang XY, Wu YS, Zhang L, Yu ZY. Corrosion. 2001;57(6):540–546. [Google Scholar]
- 51.Shabalovskaya SA. Int Mater Rev. 2001;46(5):233–250. [Google Scholar]
- 52.Snyder RG, Strauss HL. J. Pys. Chem. 1982;86:5145–5150. [Google Scholar]
- 53.Nuzzo RG, Dubois LH, Allara DL. J. Am. Chem. Soc. 1990;112:558–569. [Google Scholar]
- 54.Telegdi J, Rigo T, Kalman E. Corrosion Engineering, Science and Technology. 2004;39(1):65–70. [Google Scholar]
- 55.Deng H, Nanjo H, Qian P, Xia Z, Ishikawa I, Suzuki TM. Electrochimica Acta. 2008;53(6):2972–2983. [Google Scholar]
- 56.Faucheux A, Gouget-Laemmel AC, de Villeneuve CH, Boukherroub R, Ozanam F, Allongue P, Chazalviel JN. Langmuir. 2006;22(1):153–162. doi: 10.1021/la052145v. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, Vo AN, Barriet D, Shon YS, Lee TR. Langmuir. 2005;21(7):2902–2911. doi: 10.1021/la0475573. [DOI] [PubMed] [Google Scholar]
- 58.Hanson EL, Schwartz J, Nickel B, Koch N, Danisman MF. J Am Chem Soc. 2003;125(51):16074–16080. doi: 10.1021/ja035956z. [DOI] [PubMed] [Google Scholar]
- 59.Nakamoto K, Fugita J, Tanaka S. J. Am. Chem. Soc. 1957;79(18):4904–4908. [Google Scholar]
- 60.Liu Y, Yu Z, Zhou S, Wu L. Applied Surface Science. 2006;252(10):3818–3827. [Google Scholar]
- 61.Lin SY, Tsai TK, Lin CM, Chen CH, Chan YC, Chen HW. Langmuir. 2002;18(14):5473–5478. [Google Scholar]
- 62.Tao Y, Lin W, Hietpas GD, Allara DL. J. Pys. Chem. 1997;101:9732–9740. [Google Scholar]
- 63.Shon Y-S, Wuelfing WP, Murray RW. Langmuir. 2001;17(4):1255–1261. [Google Scholar]
- 64.Aronoff YG, Chen B, Lu G, Seto C, Schwartz J, Bernasek S. J Am Chem Soc. 1997;119(2):259–262. [Google Scholar]
- 65.McCafferty E, Wightman JP. Surf. Interface Anal. 1998;26:549–564. [Google Scholar]
- 66.Wallace RM, Chen PJ, Henck SA, Webb DA. J. Vac. Sci. Technol. A. 1995;13(3):1345–1350. [Google Scholar]
- 67.Kanicky JR, Shah DO. Special JCIS issue in honor of Prof. Somasundaran. Gainesville, FL: 2000. pp. 2–14. [Google Scholar]
- 68.Smith DA, Wallwork ML, Zhang J, Kirkham J, Robinson C, Marsh A, Wong M. J. Phys. Chem. B. 2000;104(37):8862–8870. [Google Scholar]
- 69.Zhang J, Kirkham J, Robinson C, Wallwork ML, Smith DA, Marsh A, Wong M. Anal. Chem. 2000;72(9):1973–1978. doi: 10.1021/ac9913107. [DOI] [PubMed] [Google Scholar]
- 70.Winter N, Vieceli J, Benjamin I. J. Phys. Chem. B. 2008;112(2):227–231. doi: 10.1021/jp0734833. [DOI] [PubMed] [Google Scholar]
- 71.Bordwell FG, Fried HE, Hughes DL, Lynch TY, Satish AV, Whang YE. J. Org. Chem. 1990;55(10):3330–3336. [Google Scholar]
- 72.Bohm S, Exner O. Org. Biomol. Chem. 2003;1:1176–1180. doi: 10.1039/b212298g. [DOI] [PubMed] [Google Scholar]
- 73.Ventura ON, Rama JB, Turi L, Dannenberg JJ. J. Am. Chem. Soc. 1993;115(13):5754–5761. [Google Scholar]
- 74.Putvinski TM, Schilling ML, Katz HE, Chidsey CED, Mujsce AM, Emerson AB. Langmuir. 1990;6(10):1567–1571. [Google Scholar]
- 75.Liakos IL, Newman RC, McAlpine E, Alexander MR. Surf.Interface Anal. 2004;36:347–354. [Google Scholar]
- 76.Pawsey S, McCormick M, De Paul S, Graf R, Lee YS, Reven L, Spiess HW. J Am Chem Soc. 2003;125(14):4174–4184. doi: 10.1021/ja029008u. [DOI] [PubMed] [Google Scholar]
- 77.Laibinis PE, Hickman JJ, Wrighton MS, Whitesides GM. Science. 1989;245:845. doi: 10.1126/science.245.4920.845. [DOI] [PubMed] [Google Scholar]
- 78.Wightman EMaJP. Surface and Interface Analysis. 1998;26:549–564. [Google Scholar]
- 79.Textor M, Ruiz L, Hofer R, Rossi A, Feldman K, Hahner G, Spencer ND. Langmuir. 2000;16(7):3257–3271. [Google Scholar]
- 80.Tosatti S, Michael R, Textor M, Spencer ND. Langmuir. 2002;18:3537–3548. [Google Scholar]
- 81.Gawalt ES, Avaltroni MJ, Danahy MP, Silverman BM, Hanson EL, Midwood KS, Schwarzbauer JE, Schwartz J. Langmuir. 2003;19(17):7147–7147. [Google Scholar]
- 82.Lu G, Bernasek SL, Schwartz J. Surface Science. 2000;458(1–3):80–90. [Google Scholar]
- 83.Kallay N, Torbie Z, Golie M, Matijevie E. J. Phys. Chem. 1991;95:7028–7032. [Google Scholar]
- 84.Parks GA. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Stanford, CA: Department of Mineral Engineering, Stanford University; 1964. Sep 9, pp. 177–197. 1964. [Google Scholar]
- 85.Sun CH, Berg JC. Advances in Colloid and Interface Science. 2003;105:151–175. doi: 10.1016/s0001-8686(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 86.Firstov GS, Vitchev RG, Kumar H, Blanpain B, Humbeeck JV. Biomaterials. 2002;23:4863–4871. doi: 10.1016/s0142-9612(02)00244-2. [DOI] [PubMed] [Google Scholar]
- 87.Clarke B, Carroll W, Rochev Y, Hynes M, Bradley D, Plumley D. Journal of Biomedical Materials Research Part A. 2006;79A:61–70. doi: 10.1002/jbm.a.30720. [DOI] [PubMed] [Google Scholar]
- 88.Green SM, Grant DM, Wood JV. Materials Science & Engineering A. 1997;224(1–2):21–26. [Google Scholar]
- 89.Sonnenschein MF, Cheatham CM. Langmuir. 2002;18(9):3578–3584. [Google Scholar]
- 90.Parks GA. Chem.Rev. 1965;65:177–198. [Google Scholar]
- 91.Parks GA, Bruyn PLd. J. Phys. Chem. 1962;66(6):967–973. [Google Scholar]
- 92.Moriwaki H, Yoshikawa Y, Morimoto T. Langmuir. 1990;6(4):847–850. [Google Scholar]
- 93.Takahashi K, Fukuzaki S. Biocontrol Science. 2008;13(1):9–16. doi: 10.4265/bio.13.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.