Fig. 2.
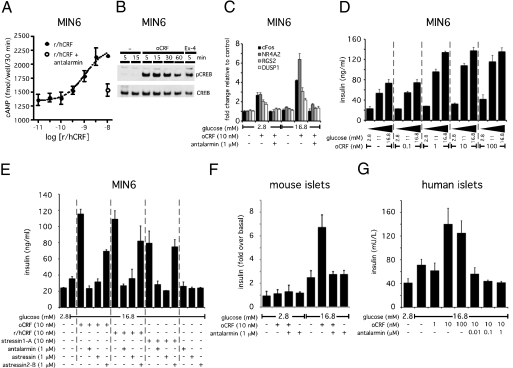
CRFR1 activation induces a cAMP response that augments GSIS in MIN6 insulinoma cells and primary rodent and human islets. CRF dose-dependently increases intracellular cAMP levels in MIN6 cells; this increase is blocked by the simultaneous administration of the CRFR1-selective antagonist antalarmin (A). Stimulation of MIN6 cells with the CRFR1-selective agonist oCRF rapidly induces robust phosphorylation of CREB (B) and leads to the stimulation of the expression of immediate-early genes including cFos, nuclear receptor subfamily 4 group A member 2 (NR4A2), regulator of G protein signaling 2 (RGS2), and dual-specificity phosphatase 1 (DUSP1) (C). These transcriptional changes are enhanced by high ambient glucose concentrations and can be blocked by the CRFR1 antagonist antalarmin. Stimulation of MIN6 cells with a relatively low dose of oCRF (1 nM) suffices to augment insulin secretion in the presence of intermediate (11 mM) or high (16.8 mM) glucose concentrations (D). The augmentation of GSIS induced by selective or preferential CRFR1 agonists (oCRF, r/hCRF, stressin1-A) is completely inhibited by general (astressin) or CRFR1-selective (antalarmin) antagonists but not by the CRFR2-selective antagonist astressin2-B (E). Stimulation with oCRF potentiates GSIS in mouse (F) and human (G) primary islets in a CRFR1-dependent manner, because coadministration of antalarmin complete blocks the effects of oCRF.