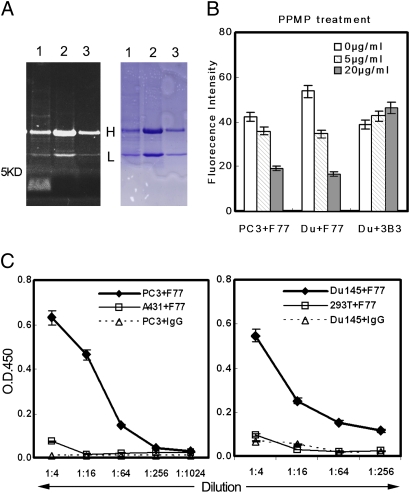
Fig. 5.
Characterization of the F77 antigen. (A) F77 Immunoprecipitation. In lane 1, 1 × 107 PC3 cells bound with F77 antibody (10 μg) were treated with 1 mL RIPA buffer at 4°C for 30 min. After centrifugation at 10,000 × g for 1 h, supernatant was incubated with protein G beads at 4°C for 1 h. In lane 2, A431 cell lysate (107 cells/1 mL RIPA buffer) was immunoprecipitated with 10 μg mAb F77. In lane 3, PC3 cell lysate (107 cells/1 mL RIPA buffer) was immunoprecipitated with 5 μg mouse IgG. Samples were separated by Tris SDS/PAGE (4–16%). Gel was first stained with Pro-Q Emerald 300 carbohydrate staining (Left) following the instructions of the manufacturer, and then stained with Coomassie blue (Right). The fluorescent signal obtained with the Pro-Q Emerald 300 stain was visualized using a 300-nm transilluminator. (B) PPMP treatment of PC3 and Du145. After treating PC3 or Du145 cells with glycosphingolipid inhibitor PPMP (0, 5, or 20 μg/mL) for 48 h, the binding of mAb F77 to cells was analyzed by flow cytometry. PPMP treatment induced a dose-dependent decrease of the F77 antigen level on both PC3 and Du145 cell surfaces. (C) Specific binding of mAb F77 to glycolipid extracted from PC3 and Du145. Glycolipid extractions by chloroform/methanol from prostate cell lines PC3 and Du145 or control cell lines HEK293 and A431 were coated onto 96-well flat-bottom plates at a serial dilution (1:4–1:1,024) for ELISA with F77 antibody (1 μg/mL). Anti-CD147 monoclonal antibody 3B3 was used as a control murine IgG3.