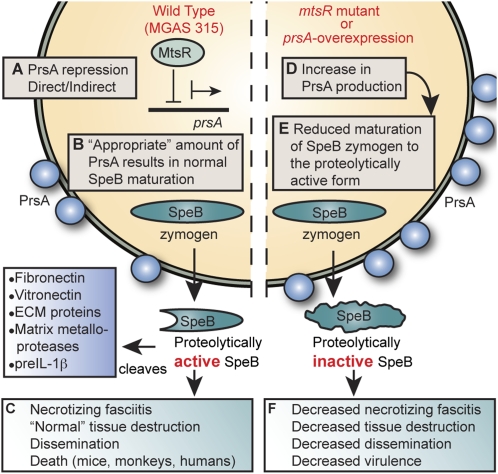
Fig. 6.
Model for the role of the mtsR-prsA-SpeB virulence axis in GAS necrotizing fasciitis. (A) In wild-type GAS strains such MGAS315, MtsR directly or indirectly regulates PrsA expression. (B) PrsA, a peptidyl-prolyl isomerase, is essential for proper maturation of the broad-spectrum cysteine protease SpeB. (C) In this wild-type condition, there is an appropriate stoichiometry between PrsA and SpeB, resulting in a normal level of extracellular SpeB cysteine protease activity and full GAS virulence. (D and E) Dysregulated overexpression of PrsA via either mtsR deletion (MGAS315ΔmtsR and MGAS9887) or overexpression of prsA transcription (MGAS315prsA-overexpress) disrupts the normal stoichiometry between PrsA and SpeB, significantly decreasing extracellular cysteine protease activity. (F) Without the wild-type level of proteolytically active SpeB available to act on its many downstream targets, the mutant GAS organisms (ΔmtsR, prsA-overexpress, and ΔspeB) are rendered considerably less virulent in muscle. They have a reduced ability to destroy soft tissue, disseminate from the infection site, and cause human necrotizing fasciitis and death.