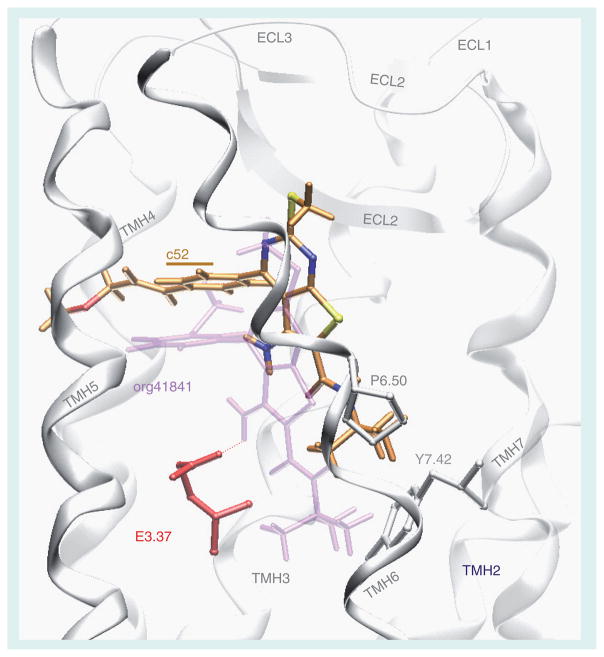
Figure 4. Comparison of docking modes of the antagonist NIDDK-CEB-52 (C atoms orange) and the partial agonist org41841 (light purple) in a model of the transmembrane helical bundle of thyroid-stimulating hormone receptor (TSHR).
The binding pocket for NIDDK-CEB-52 is within the extracellular half of the transmembrane helical bundle between helices 3, 5, 6, and 7 (white) and close to extracellular loop 2 (white). The model predicts that NIDDK-CEB-52 would not be an agonist because its amino group does not interact with the negatively charged glutamic acid in helix 3 (E3.37) that is essential for TSHR activation by org41841. In addition, in contrast to org41841, the model predicts that the t-butyl group of NIDDK-CEB-52 sits higher in the binding cleft and points toward a proline residue in helix 6 (P6.50). Therefore, movement of helix 6 during TSHR activation is not enforced by NIDDK-CEB-52. This structural constraint may cause an antagonistic effect on TSHR signaling. Our experimental findings are consistent with the model of the binding pocket.
Reproduced from [90] © US Federal Government.