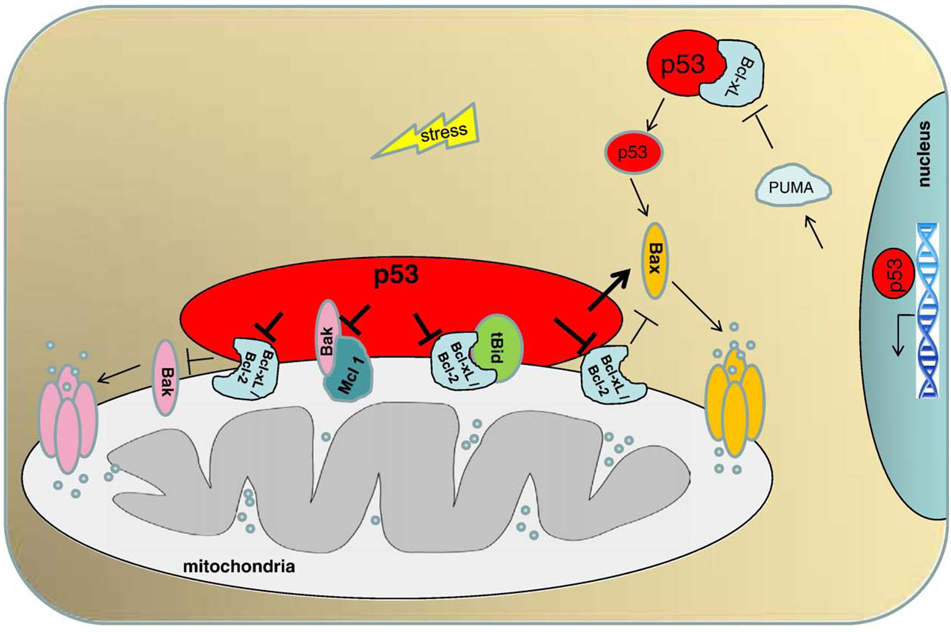
Fig. 1.
The direct mitochondrial p53 program of apoptosis. Stress-induced mitochondrial translocation of p53 results in interactions with multidomain members (anti- and proapoptotic) of the Bcl-2 family to induce mitochondrial outer membrane permeabilization. p53 interacts with Bcl-xL and Bcl2 and neutralizes their inhibitory effects on proapoptotic Bax and Bak, which are the only members of the Bcl2 family able to oligomerize and form lipid pores on the mitochondrial outer membrane. p53 interaction with Bcl-xL also liberates proapoptotic tBid from its inhibitory complex with Bcl-xL. Moreover, p53 interacts directly with Bak, liberating it from an inhibitory complex with anti-apoptotic Mcl-1. Thus, p53 acts like a ‘super’ BH3-only protein, combining both enabling and activating BH3-only functions. p53 also interacts with Bax in a ‘hit and run’ manner, which stimulates Bax oligomerization and pore formation. In an additional cytosolic p53 pathway, p53 first transactivates Puma, which then liberates p53 from a pre-existing cytosolic Bcl-xL complex to activate monomeric Bax in the cytosol.